[English] 日本語
 Yorodumi
Yorodumi- EMDB-17048: Virus-like Particle based on PVY coat protein with helical archit... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Virus-like Particle based on PVY coat protein with helical architecture encapsidating ssRNA | |||||||||
 Map data Map data | VLPhRNA shyrp symmetric cryoEM map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | helical / VLP / ssRNA / Potyvirus / PVY / VIRUS LIKE PARTICLE | |||||||||
| Function / homology | Potyvirus coat protein / Potyvirus coat protein / viral capsid / Genome polyprotein Function and homology information Function and homology information | |||||||||
| Biological species |  Potato virus Y strain NTN Potato virus Y strain NTN | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||
 Authors Authors | Kavcic L / Kezar A / Podobnik M | |||||||||
| Funding support |  Slovenia, 2 items Slovenia, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Chem / Year: 2024 Journal: Commun Chem / Year: 2024Title: From structural polymorphism to structural metamorphosis of the coat protein of flexuous filamentous potato virus Y. Authors: Luka Kavčič / Andreja Kežar / Neža Koritnik / Magda Tušek Žnidarič / Tajda Klobučar / Žiga Vičič / Franci Merzel / Ellie Holden / Justin L P Benesch / Marjetka Podobnik /   Abstract: The structural diversity and tunability of the capsid proteins (CPs) of various icosahedral and rod-shaped viruses have been well studied and exploited in the development of smart hybrid ...The structural diversity and tunability of the capsid proteins (CPs) of various icosahedral and rod-shaped viruses have been well studied and exploited in the development of smart hybrid nanoparticles. However, the potential of CPs of the wide-spread flexuous filamentous plant viruses remains to be explored. Here, we show that we can control the shape, size, RNA encapsidation ability, symmetry, stability and surface functionalization of nanoparticles through structure-based design of CP from potato virus Y (PVY). We provide high-resolution insight into CP-based self-assemblies, ranging from large polymorphic or monomorphic filaments to smaller annular, cubic or spherical particles. Furthermore, we show that we can prevent CP self-assembly in bacteria by fusion with a cleavable protein, enabling controlled nanoparticle formation in vitro. Understanding the remarkable structural diversity of PVY CP not only provides possibilities for the production of biodegradable nanoparticles, but may also advance future studies of CP's polymorphism in a biological context. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17048.map.gz emd_17048.map.gz | 23.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17048-v30.xml emd-17048-v30.xml emd-17048.xml emd-17048.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17048_fsc.xml emd_17048_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_17048.png emd_17048.png | 69.5 KB | ||
| Masks |  emd_17048_msk_1.map emd_17048_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17048.cif.gz emd-17048.cif.gz | 6.3 KB | ||
| Others |  emd_17048_additional_1.map.gz emd_17048_additional_1.map.gz emd_17048_half_map_1.map.gz emd_17048_half_map_1.map.gz emd_17048_half_map_2.map.gz emd_17048_half_map_2.map.gz | 51.7 MB 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17048 http://ftp.pdbj.org/pub/emdb/structures/EMD-17048 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17048 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17048 | HTTPS FTP |
-Related structure data
| Related structure data |  8opcMC  8opaC  8opbC  8opdC  8opeC  8opfC  8opgC  8ophC  8opjC  8opkC  8oplC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17048.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17048.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | VLPhRNA shyrp symmetric cryoEM map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.95 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17048_msk_1.map emd_17048_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
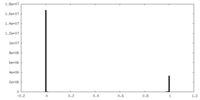
| Density Histograms |
-Additional map: VLPhRNA raw cryoEM map
| File | emd_17048_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | VLPhRNA raw cryoEM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
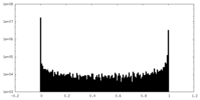
| Density Histograms |
-Half map: VLPhRNA half A cryoEM map
| File | emd_17048_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | VLPhRNA half A cryoEM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
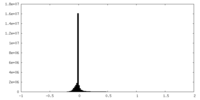
| Density Histograms |
-Half map: VLPhRNA half B cryoEM map
| File | emd_17048_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | VLPhRNA half B cryoEM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
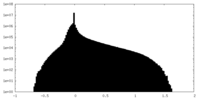
| Density Histograms |
- Sample components
Sample components
-Entire : Virus-like particle from PVY coat protein
| Entire | Name: Virus-like particle from PVY coat protein |
|---|---|
| Components |
|
-Supramolecule #1: Virus-like particle from PVY coat protein
| Supramolecule | Name: Virus-like particle from PVY coat protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: The sample contains 3 forms of filaments. |
|---|---|
| Source (natural) | Organism:  Potato virus Y strain NTN Potato virus Y strain NTN |
-Macromolecule #1: Genome polyprotein (Fragment)
| Macromolecule | Name: Genome polyprotein (Fragment) / type: protein_or_peptide / ID: 1 Details: Due to flexibility, we could not determine the 41 N-terminal residues. Number of copies: 28 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Potato virus Y strain NTN / Strain: NTN Potato virus Y strain NTN / Strain: NTN |
| Molecular weight | Theoretical: 29.935797 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GNDTIDAGGS TKKDAKQEQG SIQPNLNKEK EKDVNVGTSG THTVPRIKAI TSKMRMPKSK GATVLNLEHL LEYAPQQIDI SNTRATQSQ FDTWYEAVQL AYDIGETEMP TVMNGLMVWC IENGTSPNIN GVWVMMDGDE QVEYPLKPIV ENAKPTLRQI M AHFSDVAE ...String: GNDTIDAGGS TKKDAKQEQG SIQPNLNKEK EKDVNVGTSG THTVPRIKAI TSKMRMPKSK GATVLNLEHL LEYAPQQIDI SNTRATQSQ FDTWYEAVQL AYDIGETEMP TVMNGLMVWC IENGTSPNIN GVWVMMDGDE QVEYPLKPIV ENAKPTLRQI M AHFSDVAE AYIEMRNKKE PYMPRYGLVR NLRDGSLARY AFDFYEVTSR TPVRAREAHI QMKAAALKSA QSRLFGLDGG IS TQEENTE RHTTEDVSPS MHTLLGVKNM UniProtKB: Genome polyprotein |
-Macromolecule #2: RNA (5'-R(P*UP*UP*UP*UP*U)-3')
| Macromolecule | Name: RNA (5'-R(P*UP*UP*UP*UP*U)-3') / type: rna / ID: 2 / Number of copies: 28 |
|---|---|
| Source (natural) | Organism:  Potato virus Y strain NTN Potato virus Y strain NTN |
| Molecular weight | Theoretical: 1.485872 KDa |
| Sequence | String: UUUUU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 1.8 mM KH2PO4, 10.1 mM Na2HPO4, 140 mM NaCl, 2.7 mM KCl, pH 7.4 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number real images: 502 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 150000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)