+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the MlaCD complex (1:6 stoichiometry) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Outer membrane / gram-negative bacteria / lipid transfer / antibiotic resistance / LIPID BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationphospholipid transfer activity / intermembrane phospholipid transfer / phospholipid-translocating ATPase complex / phospholipid transport / phospholipid binding / outer membrane-bounded periplasmic space / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.35 Å | |||||||||
 Authors Authors | Wotherspoon P / Bui S / Sridhar P / Bergeron JRC / Knowles TJ | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structure of the MlaC-MlaD complex reveals molecular basis of periplasmic phospholipid transport. Authors: Peter Wotherspoon / Hannah Johnston / David J Hardy / Rachel Holyfield / Soi Bui / Giedrė Ratkevičiūtė / Pooja Sridhar / Jonathan Colburn / Charlotte B Wilson / Adam Colyer / Benjamin F ...Authors: Peter Wotherspoon / Hannah Johnston / David J Hardy / Rachel Holyfield / Soi Bui / Giedrė Ratkevičiūtė / Pooja Sridhar / Jonathan Colburn / Charlotte B Wilson / Adam Colyer / Benjamin F Cooper / Jack A Bryant / Gareth W Hughes / Phillip J Stansfeld / Julien R C Bergeron / Timothy J Knowles /  Abstract: The Maintenance of Lipid Asymmetry (Mla) pathway is a multicomponent system found in all gram-negative bacteria that contributes to virulence, vesicle blebbing and preservation of the outer membrane ...The Maintenance of Lipid Asymmetry (Mla) pathway is a multicomponent system found in all gram-negative bacteria that contributes to virulence, vesicle blebbing and preservation of the outer membrane barrier function. It acts by removing ectopic lipids from the outer leaflet of the outer membrane and returning them to the inner membrane through three proteinaceous assemblies: the MlaA-OmpC complex, situated within the outer membrane; the periplasmic phospholipid shuttle protein, MlaC; and the inner membrane ABC transporter complex, MlaFEDB, proposed to be the founding member of a structurally distinct ABC superfamily. While the function of each component is well established, how phospholipids are exchanged between components remains unknown. This stands as a major roadblock in our understanding of the function of the pathway, and in particular, the role of ATPase activity of MlaFEDB is not clear. Here, we report the structure of E. coli MlaC in complex with the MlaD hexamer in two distinct stoichiometries. Utilising in vivo complementation assays, an in vitro fluorescence-based transport assay, and molecular dynamics simulations, we confirm key residues, identifying the MlaD β6-β7 loop as essential for MlaCD function. We also provide evidence that phospholipids pass between the C-terminal helices of the MlaD hexamer to reach the central pore, providing insight into the trajectory of GPL transfer between MlaC and MlaD. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16904.map.gz emd_16904.map.gz | 154.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16904-v30.xml emd-16904-v30.xml emd-16904.xml emd-16904.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16904.png emd_16904.png | 32.6 KB | ||
| Filedesc metadata |  emd-16904.cif.gz emd-16904.cif.gz | 6.4 KB | ||
| Others |  emd_16904_half_map_1.map.gz emd_16904_half_map_1.map.gz emd_16904_half_map_2.map.gz emd_16904_half_map_2.map.gz | 151.6 MB 151.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16904 http://ftp.pdbj.org/pub/emdb/structures/EMD-16904 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16904 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16904 | HTTPS FTP |
-Related structure data
| Related structure data |  8oj4MC  8ojgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16904.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16904.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.71 Å | ||||||||||||||||||||||||||||||||||||
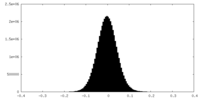
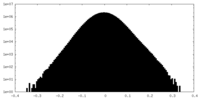
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_16904_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
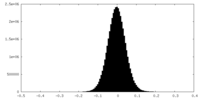
| Density Histograms |
-Half map: #1
| File | emd_16904_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
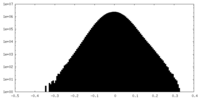
| Density Histograms |
- Sample components
Sample components
-Entire : MlaCD
| Entire | Name: MlaCD |
|---|---|
| Components |
|
-Supramolecule #1: MlaCD
| Supramolecule | Name: MlaCD / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Intermembrane phospholipid transport system binding protein MlaC
| Macromolecule | Name: Intermembrane phospholipid transport system binding protein MlaC type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.989559 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFKRLMMVAL LVIAPLSAAT AADQTNPYKL MDEAAQKTFD RLKNEQPQIR ANPDYLRTIV DQELLPYVQV KYAGALVLGQ YYKSATPAQ REAYFAAFRE YLKQAYGQAL AMYHGQTYQI APEQPLGDKT IVPIRVTIID PNGRPPVRLD FQWRKNSQTG N WQAYDMIA ...String: MFKRLMMVAL LVIAPLSAAT AADQTNPYKL MDEAAQKTFD RLKNEQPQIR ANPDYLRTIV DQELLPYVQV KYAGALVLGQ YYKSATPAQ REAYFAAFRE YLKQAYGQAL AMYHGQTYQI APEQPLGDKT IVPIRVTIID PNGRPPVRLD FQWRKNSQTG N WQAYDMIA EGVSMITTKQ NEWGTLLRTK GIDGLTAQLK SISQQKITLE EKK UniProtKB: Intermembrane phospholipid transport system binding protein MlaC |
-Macromolecule #2: Intermembrane phospholipid transport system binding protein MlaD
| Macromolecule | Name: Intermembrane phospholipid transport system binding protein MlaD type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 19.593133 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQTKKNEIWV GIFLLAALLA ALFVCLKAAN VTSIRTEPTY TLYATFDNIG GLKARSPVSI GGVVVGRVAD ITLDPKTYLP RVTLEIEQR YNHIPDTSSL SIRTSGLLGE QYLALNVGFE DPELGTAILK DGDTIQDTKS AMVLEDLIGQ FLYGSKGDDN K NSGDAPAA APGNNETTEP VGTTK UniProtKB: Intermembrane phospholipid transport system binding protein MlaD |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)