[English] 日本語
 Yorodumi
Yorodumi- EMDB-16672: Pyruvate dehydrogenase complex core (E2, dihydrolipoyl transacetylase) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pyruvate dehydrogenase complex core (E2, dihydrolipoyl transacetylase) | |||||||||
 Map data Map data | Pyruvate dehydrogenase complex core | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transferase / dodecahedron / mitochondrion | |||||||||
| Biological species |  | |||||||||
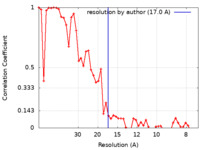
| Method | subtomogram averaging / cryo EM / Resolution: 17.0 Å | |||||||||
 Authors Authors | Plokhikh KS / Chesnokov YM | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: FEBS J / Year: 2024 Journal: FEBS J / Year: 2024Title: Association of 2-oxoacid dehydrogenase complexes with respirasomes in mitochondria. Authors: Konstantin S Plokhikh / Semen V Nesterov / Yuriy M Chesnokov / Anton G Rogov / Roman A Kamyshinsky / Aleksandr L Vasiliev / Lev S Yaguzhinsky / Raif G Vasilov /  Abstract: In the present study, cryo-electron tomography was used to investigate the localization of 2-oxoacid dehydrogenase complexes (OADCs) in cardiac mitochondria and mitochondrial inner membrane samples. ...In the present study, cryo-electron tomography was used to investigate the localization of 2-oxoacid dehydrogenase complexes (OADCs) in cardiac mitochondria and mitochondrial inner membrane samples. Two classes of ordered OADC inner cores with different symmetries were distinguished and their quaternary structures modeled. One class corresponds to pyruvate dehydrogenase complexes and the other to dehydrogenase complexes of α-ketoglutarate and branched-chain α-ketoacids. OADCs were shown to be localized in close proximity to membrane-embedded respirasomes, as observed both in densely packed lamellar cristae of cardiac mitochondria and in ruptured mitochondrial samples where the dense packing is absent. This suggests the specificity of the OADC-respirasome interaction, which allows localized NADH/NAD exchange between OADCs and complex I of the respiratory chain. The importance of this local coupling is based on OADCs being the link between respiration, glycolysis and amino acid metabolism. The coupling of these basic metabolic processes can vary in different tissues and conditions and may be involved in the development of various pathologies. The present study shows that this important and previously missing parameter of mitochondrial complex coupling can be successfully assessed using cryo-electron tomography. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16672.map.gz emd_16672.map.gz | 781.5 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16672-v30.xml emd-16672-v30.xml emd-16672.xml emd-16672.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16672_fsc.xml emd_16672_fsc.xml | 5.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_16672.png emd_16672.png | 199 KB | ||
| Masks |  emd_16672_msk_1.map emd_16672_msk_1.map | 8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16672.cif.gz emd-16672.cif.gz | 5.2 KB | ||
| Others |  emd_16672_half_map_1.map.gz emd_16672_half_map_1.map.gz emd_16672_half_map_2.map.gz emd_16672_half_map_2.map.gz | 5.9 MB 5.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16672 http://ftp.pdbj.org/pub/emdb/structures/EMD-16672 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16672 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16672 | HTTPS FTP |
-Validation report
| Summary document |  emd_16672_validation.pdf.gz emd_16672_validation.pdf.gz | 514.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16672_full_validation.pdf.gz emd_16672_full_validation.pdf.gz | 514.3 KB | Display | |
| Data in XML |  emd_16672_validation.xml.gz emd_16672_validation.xml.gz | 10.8 KB | Display | |
| Data in CIF |  emd_16672_validation.cif.gz emd_16672_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16672 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16672 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16672 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16672 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16672.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16672.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Pyruvate dehydrogenase complex core | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.7 Å | ||||||||||||||||||||||||||||||||||||
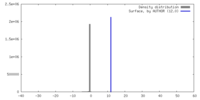
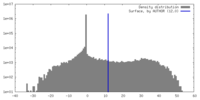
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16672_msk_1.map emd_16672_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
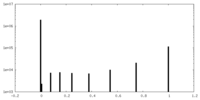
| Density Histograms |
-Half map: #2
| File | emd_16672_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
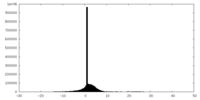
| Density Histograms |
-Half map: #1
| File | emd_16672_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ruptured mitochondrial membrane samples
| Entire | Name: ruptured mitochondrial membrane samples |
|---|---|
| Components |
|
-Supramolecule #1: ruptured mitochondrial membrane samples
| Supramolecule | Name: ruptured mitochondrial membrane samples / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: dihydrolipoyl transacetylase
| Macromolecule | Name: dihydrolipoyl transacetylase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue acetyltransferase |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MWRVCARRVQ SAVPRAGFRA RWATLKGPRT GPAAVRCGSG IPSYGVRSLC GWSYGSATVP RNRILQQLLG SPSRRSYSLP PHQKVPLPSL SPTMQAGTIA RWEKKEGEKI SEGDLIAEVE TDKATVGFES LEECYMAKIL VPEGTRDVPV GSIICITVEK PQDIEAFKNY ...String: MWRVCARRVQ SAVPRAGFRA RWATLKGPRT GPAAVRCGSG IPSYGVRSLC GWSYGSATVP RNRILQQLLG SPSRRSYSLP PHQKVPLPSL SPTMQAGTIA RWEKKEGEKI SEGDLIAEVE TDKATVGFES LEECYMAKIL VPEGTRDVPV GSIICITVEK PQDIEAFKNY TLDSATAATQ AAPAPAAAPA AAPAAPSASA PGSSYPVHMQ IVLPALSPTM TMGTVQRWEK KVGEKLSEGD LLAEIETDKA TIGFEVQEEG YLAKILVPEG TRDVPLGTPL CIIVEKQEDI AAFADYRPTE VTSLKPQAPP PVPPPVAAVP PIPQPLAPTP SAAPAGPKGR VFVSPLAKKL AAEKGIDLTQ VKGTGPEGRI IKKDIDSFVP TKAAPAAAAA APPGPRVAPT PAGVFIDIPI SNIRRVIAQR LMQSKQTIPH YYLSVDVNMG EVLLVRKELN KMLEGKGKIS VNDFIIKASA LACLKVPEAN SSWMDTVIRQ NHVVDVSVAV STPAGLITPI VFNAHIKGLE TIASDVVSLA SKAREGKLQP HEFQGGTFTI SNLGMFGIKN FSAIINPPQA CILAIGASED KLIPADNEKG FDVASVMSVT LSCDHRVVDG AVGAQWLAEF KKYLEKPVTM LL |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Component - Concentration: 10.0 mM / Component - Name: Hepes/KOH |
|---|---|
| Grid | Model: EMS Lacey Carbon / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.026000000000000002 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 1.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 8.0 µm / Nominal defocus min: 6.0 µm / Nominal magnification: 18000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)