+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | cryo-EM Structure of Craf:14-3-3:Mek1 | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Kinase / STRUCTURAL PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報death-inducing signaling complex assembly / epithelial cell proliferation involved in lung morphogenesis / positive regulation of endodermal cell differentiation / negative regulation of homotypic cell-cell adhesion / negative regulation of hypoxia-induced intrinsic apoptotic signaling pathway / regulation of vascular associated smooth muscle contraction / intermediate filament cytoskeleton organization / mitogen-activated protein kinase kinase / placenta blood vessel development / positive regulation of muscle contraction ...death-inducing signaling complex assembly / epithelial cell proliferation involved in lung morphogenesis / positive regulation of endodermal cell differentiation / negative regulation of homotypic cell-cell adhesion / negative regulation of hypoxia-induced intrinsic apoptotic signaling pathway / regulation of vascular associated smooth muscle contraction / intermediate filament cytoskeleton organization / mitogen-activated protein kinase kinase / placenta blood vessel development / positive regulation of muscle contraction / Golgi inheritance / MAP-kinase scaffold activity / regulation of axon regeneration / cerebellar cortex formation / labyrinthine layer development / regulation of Rho protein signal transduction / melanosome transport / type B pancreatic cell proliferation / Signaling by MAP2K mutants / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / Rap1 signalling / vesicle transport along microtubule / positive regulation of axonogenesis / positive regulation of Ras protein signal transduction / insulin secretion involved in cellular response to glucose stimulus / regulation of Golgi inheritance / mitogen-activated protein kinase kinase kinase binding / central nervous system neuron differentiation / triglyceride homeostasis / trachea formation / Negative feedback regulation of MAPK pathway / regulation of early endosome to late endosome transport / IFNG signaling activates MAPKs / regulation of stress-activated MAPK cascade / Frs2-mediated activation / GP1b-IX-V activation signalling / MAPK3 (ERK1) activation / ERBB2-ERBB3 signaling pathway / neurotrophin TRK receptor signaling pathway / regulation of neurotransmitter receptor localization to postsynaptic specialization membrane / face development / pseudopodium / endodermal cell differentiation / MAP kinase kinase activity / Bergmann glial cell differentiation / positive regulation of ATP biosynthetic process / regulation of cell differentiation / thyroid gland development / Uptake and function of anthrax toxins / positive regulation of protein serine/threonine kinase activity / extrinsic apoptotic signaling pathway via death domain receptors / somatic stem cell population maintenance / positive regulation of peptidyl-serine phosphorylation / MAP kinase kinase kinase activity / protein kinase activator activity / type II interferon-mediated signaling pathway / Schwann cell development / response to axon injury / negative regulation of protein-containing complex assembly / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / keratinocyte differentiation / neuron projection morphogenesis / response to muscle stretch / ERK1 and ERK2 cascade / myelination / protein serine/threonine/tyrosine kinase activity / positive regulation of autophagy / CD209 (DC-SIGN) signaling / dendrite cytoplasm / insulin-like growth factor receptor signaling pathway / response to glucocorticoid / thymus development / MAP3K8 (TPL2)-dependent MAPK1/3 activation / adenylate cyclase activator activity / protein serine/threonine kinase activator activity / Signal transduction by L1 / cell motility / positive regulation of transcription elongation by RNA polymerase II / wound healing / RAF activation / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / small GTPase binding / Stimuli-sensing channels / neuron differentiation / chemotaxis / Negative regulation of MAPK pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / cellular senescence / Signaling by BRAF and RAF1 fusions / insulin receptor signaling pathway / late endosome / MAPK cascade / heart development / response to oxidative stress / protein tyrosine kinase activity 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
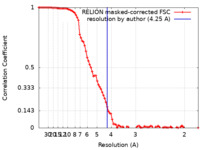
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 4.25 Å | |||||||||
 データ登録者 データ登録者 | Dedden D / Ulrich G | |||||||||
| 資金援助 |  ドイツ, 1件 ドイツ, 1件
| |||||||||
 引用 引用 |  ジャーナル: J Mol Biol / 年: 2024 ジャーナル: J Mol Biol / 年: 2024タイトル: Cryo-EM Structures of CRAF/14-3-3 and CRAF/14-3-3/MEK1 Complexes. 著者: Dirk Dedden / Julius Nitsche / Elisabeth V Schneider / Maren Thomsen / Daniel Schwarz / Birgitta Leuthner / Ulrich Grädler /  要旨: RAF protein kinases are essential effectors in the MAPK pathway and are important cancer drug targets. Structural understanding of RAF activation is so far based on cryo-electron microscopy (cryo-EM) ...RAF protein kinases are essential effectors in the MAPK pathway and are important cancer drug targets. Structural understanding of RAF activation is so far based on cryo-electron microscopy (cryo-EM) and X-ray structures of BRAF in different conformational states as inactive or active complexes with KRAS, 14-3-3 and MEK1. In this study, we have solved the first cryo-EM structures of CRAF/14-3-3 at 3.4 Å resolution and CRAF/14-3-3/MEK1 at 4.2 Å resolution using CRAF kinase domain expressed as constitutively active Y340D/Y341D mutant in insect cells. The overall architecture of our CRAF/14-3-3 and CRAF/14-3-3/MEK1 cryo-EM structures is highly similar to corresponding BRAF structures in complex with 14-3-3 or 14-3-3/MEK1 and represent the activated dimeric RAF conformation. Our CRAF cryo-EM structures provide additional insights into structural understanding of the activated CRAF/14-3-3/MEK1 complex. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_16660.map.gz emd_16660.map.gz | 55.2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-16660-v30.xml emd-16660-v30.xml emd-16660.xml emd-16660.xml | 18.5 KB 18.5 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_16660_fsc.xml emd_16660_fsc.xml | 9.2 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_16660.png emd_16660.png | 65.2 KB | ||
| Filedesc metadata |  emd-16660.cif.gz emd-16660.cif.gz | 6.9 KB | ||
| その他 |  emd_16660_half_map_1.map.gz emd_16660_half_map_1.map.gz emd_16660_half_map_2.map.gz emd_16660_half_map_2.map.gz | 48.7 MB 48.7 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16660 http://ftp.pdbj.org/pub/emdb/structures/EMD-16660 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16660 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16660 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_16660_validation.pdf.gz emd_16660_validation.pdf.gz | 823 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_16660_full_validation.pdf.gz emd_16660_full_validation.pdf.gz | 822.6 KB | 表示 | |
| XML形式データ |  emd_16660_validation.xml.gz emd_16660_validation.xml.gz | 16 KB | 表示 | |
| CIF形式データ |  emd_16660_validation.cif.gz emd_16660_validation.cif.gz | 21.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16660 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16660 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16660 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16660 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8chfMC  8cpdC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_16660.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_16660.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.91366 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_16660_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
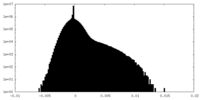
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_16660_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Complex of dimer phosphorylated C-raf kinase domain with 14-3-3 d...
| 全体 | 名称: Complex of dimer phosphorylated C-raf kinase domain with 14-3-3 dimer and 2 subunits of Mek1 containing ligand GDC-0623 |
|---|---|
| 要素 |
|
-超分子 #1: Complex of dimer phosphorylated C-raf kinase domain with 14-3-3 d...
| 超分子 | 名称: Complex of dimer phosphorylated C-raf kinase domain with 14-3-3 dimer and 2 subunits of Mek1 containing ligand GDC-0623 タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#3 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: RAF proto-oncogene serine/threonine-protein kinase
| 分子 | 名称: RAF proto-oncogene serine/threonine-protein kinase / タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO / EC番号: non-specific serine/threonine protein kinase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 73.184477 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MEHIQGAWKT ISNGFGFKDA VFDGSSCISP TIVQQFGYQR RASDDGKLTD PSKTSNTIRV FLPNKQRTVV NVRNGMSLHD CLMKALKVR GLQPECCAVF RLLHEHKGKK ARLDWNTDAA SLIGEELQVD FLDHVPLTTH NFARKTFLKL AFCDICQKFL L NGFRCQTC ...文字列: MEHIQGAWKT ISNGFGFKDA VFDGSSCISP TIVQQFGYQR RASDDGKLTD PSKTSNTIRV FLPNKQRTVV NVRNGMSLHD CLMKALKVR GLQPECCAVF RLLHEHKGKK ARLDWNTDAA SLIGEELQVD FLDHVPLTTH NFARKTFLKL AFCDICQKFL L NGFRCQTC GYKFHEHCST KVPTMCVDWS NIRQLLLFPN STIGDSGVPA LPSLTMRRMR ESVSRMPVSS QHRYSTPHAF TF NTSSPSS EGSLSQRQRS TSTPNVHMVS TTLPVDSRMI EDAIRSHSES ASPSALSSSP NNLSPTGWSQ PKTPVPAQRE RAP VSGTQE KNKIRPRGQR DSSYDWEIEA SEVMLSTRIG SGSFGTVYKG KWHGDVAVKI LKVVDPTPEQ FQAFRNEVAV LRKT RHVNI LLFMGYMTKD NLAIVTQWCE GSSLYKHLHV QETKFQMFQL IDIARQTAQG MDYLHAKNII HRDMKSNNIF LHEGL TVKI GDFGLATVKS RWSGSQQVEQ PTGSVLWMAP EVIRMQDNNP FSFQSDVYSY GIVLYELMTG ELPYSHINNR DQIIFM VGR GYASPDLSKL YKNCPKAMKR LVADCVKKVK EERPLFPQIL SSIELLQHSL PKINRSA(SEP)EP SLHRAAHTED INA CTLTTS PRLPVF UniProtKB: RAF proto-oncogene serine/threonine-protein kinase |
-分子 #2: 14-3-3 protein zeta isoform X1
| 分子 | 名称: 14-3-3 protein zeta isoform X1 / タイプ: protein_or_peptide / ID: 2 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 28.108514 KDa |
| 配列 | 文字列: MSVDKEELVQ RAKLAEQAER YDDMAAAMKE VTETGVELSN EERNLLSVAY KNVVGARRSS WRVISSIEQK TEGSERKQQM AKEYRVKVE KELREICYDV LGLLDKHLIP KASNPESKVF YLKMKGDYYR YLAEVATGET RNSVVEDSQK AYQDAFEISK A KMQPTHPI ...文字列: MSVDKEELVQ RAKLAEQAER YDDMAAAMKE VTETGVELSN EERNLLSVAY KNVVGARRSS WRVISSIEQK TEGSERKQQM AKEYRVKVE KELREICYDV LGLLDKHLIP KASNPESKVF YLKMKGDYYR YLAEVATGET RNSVVEDSQK AYQDAFEISK A KMQPTHPI RLGLALNFSV FYYEILNSPD KACQLAKQAF DDAIAELDTL NEDSYKDSTL IMQLLRDNLT LWTSDTQGDG DE PAEGGDN UniProtKB: 14-3-3 protein zeta |
-分子 #3: Dual specificity mitogen-activated protein kinase kinase 1
| 分子 | 名称: Dual specificity mitogen-activated protein kinase kinase 1 タイプ: protein_or_peptide / ID: 3 / コピー数: 2 / 光学異性体: LEVO / EC番号: mitogen-activated protein kinase kinase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 43.461938 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MPKKKPTPIQ LNPAPDGSAV NGTSSAETNL EALQKKLEEL ELDEQQRKRL EAFLTQKQKV GELKDDDFEK ISELGAGNGG VVFKVSHKP SGLVMARKLI HLEIKPAIRN QIIRELQVLH ECNSPYIVGF YGAFYSDGEI SICMEHMDGG SLDQVLKKAG R IPEQILGK ...文字列: MPKKKPTPIQ LNPAPDGSAV NGTSSAETNL EALQKKLEEL ELDEQQRKRL EAFLTQKQKV GELKDDDFEK ISELGAGNGG VVFKVSHKP SGLVMARKLI HLEIKPAIRN QIIRELQVLH ECNSPYIVGF YGAFYSDGEI SICMEHMDGG SLDQVLKKAG R IPEQILGK VSIAVIKGLT YLREKHKIMH RDVKPSNILV NSRGEIKLCD FGVSGQLIDA MANAFVGTRS YMSPERLQGT HY SVQSDIW SMGLSLVEMA VGRYPIPPPD AKELELMFGC QVEGDAAETP PRPRTPGRPL SSYGMDSRPP MAIFELLDYI VNE PPPKLP SGVFSLEFQD FVNKCLIKNP AERADLKQLM VHAFIKRSDA EEVDFAGWLC STIGLNQPST PTHAAGV UniProtKB: Dual specificity mitogen-activated protein kinase kinase 1 |
-分子 #4: 2-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-3-(pyridin...
| 分子 | 名称: 2-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-3-(pyridin-4-yl)-1H-pyrazol-1-yl}ethanol タイプ: ligand / ID: 4 / コピー数: 2 / 式: 29L |
|---|---|
| 分子量 | 理論値: 334.372 Da |
| Chemical component information | 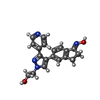 ChemComp-29L: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 0.4 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: COPPER / メッシュ: 300 / 前処理 - タイプ: GLOW DISCHARGE |
| 凍結 | 凍結剤: ETHANE-PROPANE / チャンバー内湿度: 100 % / チャンバー内温度: 298 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | TFS GLACIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: FEI FALCON IV (4k x 4k) デジタル化 - サイズ - 横: 4096 pixel / デジタル化 - サイズ - 縦: 4096 pixel / 撮影したグリッド数: 1 / 実像数: 2732 / 平均露光時間: 7.0 sec. / 平均電子線量: 60.0 e/Å2 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 50.0 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm 最大 デフォーカス(公称値): 2.3000000000000003 µm 最小 デフォーカス(公称値): 0.7000000000000001 µm 倍率(公称値): 150000 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | プロトコル: RIGID BODY FIT |
|---|---|
| 得られたモデル |  PDB-8chf: |
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)