+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CAND1 b-hairpin++-SCF-SKP2 CAND1 rolling SCF engaged | |||||||||
 Map data Map data | deepEMhancer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cullin-RING E3 ubiquitin ligase / SCF / CAND1 / Assembly factor / LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationSCF complex assembly / positive regulation of protein polyubiquitination / negative regulation of catalytic activity / Parkin-FBXW7-Cul1 ubiquitin ligase complex / cellular response to cell-matrix adhesion / F-box domain binding / Aberrant regulation of mitotic exit in cancer due to RB1 defects / PcG protein complex / negative regulation of beige fat cell differentiation / cullin-RING-type E3 NEDD8 transferase ...SCF complex assembly / positive regulation of protein polyubiquitination / negative regulation of catalytic activity / Parkin-FBXW7-Cul1 ubiquitin ligase complex / cellular response to cell-matrix adhesion / F-box domain binding / Aberrant regulation of mitotic exit in cancer due to RB1 defects / PcG protein complex / negative regulation of beige fat cell differentiation / cullin-RING-type E3 NEDD8 transferase / NEDD8 transferase activity / positive regulation of ubiquitin protein ligase activity / cullin-RING ubiquitin ligase complex / Cul7-RING ubiquitin ligase complex / maintenance of protein location in nucleus / cellular response to chemical stress / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / protein K27-linked ubiquitination / positive regulation of protein autoubiquitination / RNA polymerase II transcription initiation surveillance / protein neddylation / NEDD8 ligase activity / VCB complex / negative regulation of response to oxidative stress / Cul5-RING ubiquitin ligase complex / ubiquitin-ubiquitin ligase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / SCF ubiquitin ligase complex / Cul2-RING ubiquitin ligase complex / negative regulation of type I interferon production / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / positive regulation of intracellular estrogen receptor signaling pathway / Cul3-RING ubiquitin ligase complex / negative regulation of mitophagy / Cul4A-RING E3 ubiquitin ligase complex / Cul4-RING E3 ubiquitin ligase complex / Prolactin receptor signaling / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / cullin family protein binding / positive regulation of RNA polymerase II transcription preinitiation complex assembly / protein K63-linked ubiquitination / protein monoubiquitination / ubiquitin ligase complex / site of DNA damage / protein K48-linked ubiquitination / ubiquitin-like ligase-substrate adaptor activity / positive regulation of double-strand break repair via homologous recombination / : / signal transduction in response to DNA damage / Nuclear events stimulated by ALK signaling in cancer / transcription-coupled nucleotide-excision repair / positive regulation of TORC1 signaling / regulation of cellular response to insulin stimulus / positive regulation of smooth muscle cell proliferation / negative regulation of insulin receptor signaling pathway / intrinsic apoptotic signaling pathway / post-translational protein modification / TBP-class protein binding / molecular function activator activity / T cell activation / negative regulation of canonical NF-kappaB signal transduction / animal organ morphogenesis / Regulation of BACH1 activity / MAP3K8 (TPL2)-dependent MAPK1/3 activation / Degradation of CRY and PER proteins / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / cellular response to amino acid stimulus / Vpu mediated degradation of CD4 / Degradation of DVL / Dectin-1 mediated noncanonical NF-kB signaling / Activation of NF-kappaB in B cells / G1/S transition of mitotic cell cycle / Degradation of GLI1 by the proteasome / Iron uptake and transport / negative regulation of canonical Wnt signaling pathway / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Negative regulation of NOTCH4 signaling / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / Hedgehog 'on' state / Recognition of DNA damage by PCNA-containing replication complex / Vif-mediated degradation of APOBEC3G / APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / beta-catenin binding / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / DNA Damage Recognition in GG-NER / Evasion by RSV of host interferon responses / NOTCH1 Intracellular Domain Regulates Transcription / CLEC7A (Dectin-1) signaling / G2/M transition of mitotic cell cycle / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / SCF(Skp2)-mediated degradation of p27/p21 / Dual Incision in GG-NER Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Baek K / Schulman BA | |||||||||
| Funding support |  Germany, European Union, 2 items Germany, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Systemwide disassembly and assembly of SCF ubiquitin ligase complexes. Authors: Kheewoong Baek / Daniel C Scott / Lukas T Henneberg / Moeko T King / Matthias Mann / Brenda A Schulman /   Abstract: Cells respond to environmental cues by remodeling their inventories of multiprotein complexes. Cellular repertoires of SCF (SKP1-CUL1-F box protein) ubiquitin ligase complexes, which mediate much ...Cells respond to environmental cues by remodeling their inventories of multiprotein complexes. Cellular repertoires of SCF (SKP1-CUL1-F box protein) ubiquitin ligase complexes, which mediate much protein degradation, require CAND1 to distribute the limiting CUL1 subunit across the family of ∼70 different F box proteins. Yet, how a single factor coordinately assembles numerous distinct multiprotein complexes remains unknown. We obtained cryo-EM structures of CAND1-bound SCF complexes in multiple states and correlated mutational effects on structures, biochemistry, and cellular assays. The data suggest that CAND1 clasps idling catalytic domains of an inactive SCF, rolls around, and allosterically rocks and destabilizes the SCF. New SCF production proceeds in reverse, through SKP1-F box allosterically destabilizing CAND1. The CAND1-SCF conformational ensemble recycles CUL1 from inactive complexes, fueling mixing and matching of SCF parts for E3 activation in response to substrate availability. Our data reveal biogenesis of a predominant family of E3 ligases, and the molecular basis for systemwide multiprotein complex assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16575.map.gz emd_16575.map.gz | 111.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16575-v30.xml emd-16575-v30.xml emd-16575.xml emd-16575.xml | 28.3 KB 28.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16575_fsc.xml emd_16575_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_16575.png emd_16575.png | 136.8 KB | ||
| Masks |  emd_16575_msk_1.map emd_16575_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16575.cif.gz emd-16575.cif.gz | 8.2 KB | ||
| Others |  emd_16575_additional_1.map.gz emd_16575_additional_1.map.gz emd_16575_additional_2.map.gz emd_16575_additional_2.map.gz emd_16575_half_map_1.map.gz emd_16575_half_map_1.map.gz emd_16575_half_map_2.map.gz emd_16575_half_map_2.map.gz | 98 MB 13.6 MB 98.5 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16575 http://ftp.pdbj.org/pub/emdb/structures/EMD-16575 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16575 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16575 | HTTPS FTP |
-Related structure data
| Related structure data |  8cdjMC  7z8rC  7z8tC  7z8vC  7zbwC  7zbzC  8cdkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16575.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16575.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deepEMhancer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8512 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16575_msk_1.map emd_16575_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: 3d refinement
| File | emd_16575_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3d refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: relion postprocess
| File | emd_16575_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | relion postprocess | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: halfmap1
| File | emd_16575_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: halfmap2
| File | emd_16575_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
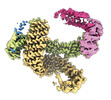
-Entire : CAND1 b-hairpin++-SCF-SKP2 CAND1 rolling SCF engaged
| Entire | Name: CAND1 b-hairpin++-SCF-SKP2 CAND1 rolling SCF engaged |
|---|---|
| Components |
|
-Supramolecule #1: CAND1 b-hairpin++-SCF-SKP2 CAND1 rolling SCF engaged
| Supramolecule | Name: CAND1 b-hairpin++-SCF-SKP2 CAND1 rolling SCF engaged / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Cullin-1
| Macromolecule | Name: Cullin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 89.800367 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSSTRSQNPH GLKQIGLDQI WDDLRAGIQQ VYTRQSMAKS RYMELYTHVY NYCTSVHQSN QARGAGVPPS KSKKGQTPGG AQFVGLELY KRLKEFLKNY LTNLLKDGED LMDESVLKFY TQQWEDYRFS SKVLNGICAY LNRHWVRREC DEGRKGIYEI Y SLALVTWR ...String: MSSTRSQNPH GLKQIGLDQI WDDLRAGIQQ VYTRQSMAKS RYMELYTHVY NYCTSVHQSN QARGAGVPPS KSKKGQTPGG AQFVGLELY KRLKEFLKNY LTNLLKDGED LMDESVLKFY TQQWEDYRFS SKVLNGICAY LNRHWVRREC DEGRKGIYEI Y SLALVTWR DCLFRPLNKQ VTNAVLKLIE KERNGETINT RLISGVVQSY VELGLNEDDA FAKGPTLTVY KESFESQFLA DT ERFYTRE STEFLQQNPV TEYMKKAEAR LLEEQRRVQV YLHESTQDEL ARKCEQVLIE KHLEIFHTEF QNLLDADKNE DLG RMYNLV SRIQDGLGEL KKLLETHIHN QGLAAIEKCG EAALNDPKMY VQTVLDVHKK YNALVMSAFN NDAGFVAALD KACG RFINN NAVTKMAQSS SKSPELLARY CDSLLKKSSK NPEEAELEDT LNQVMVVFKY IEDKDVFQKF YAKMLAKRLV HQNSA SDDA EASMISKLKQ ACGFEYTSKL QRMFQDIGVS KDLNEQFKKH LTNSEPLDLD FSIQVLSSGS WPFQQSCTFA LPSELE RSY QRFTAFYASR HSGRKLTWLY QLSKGELVTN CFKNRYTLQA STFQMAILLQ YNTEDAYTVQ QLTDSTQIKM DILAQVL QI LLKSKLLVLE DENANVDEVE LKPDTLIKLY LGYKNKKLRV NINVPMKTEQ KQEQETTHKN IEEDRKLLIQ AAIVRIMK M RKVLKHQQLL GEVLTQLSSR FKPRVPVIKK CIDILIEKEY LERVDGEKDT YSYLA UniProtKB: Cullin-1 |
-Macromolecule #2: E3 ubiquitin-protein ligase RBX1, N-terminally processed
| Macromolecule | Name: E3 ubiquitin-protein ligase RBX1, N-terminally processed type: protein_or_peptide / ID: 2 / Details: RING domain not visible / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.089677 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GSMDVDTPSG TNSGAGKKRF EVKKWNAVAL WAWDIVVDNC AICRNHIMDL CIECQANQAS ATSEECTVAW GVCNHAFHFH CISRWLKTR QVCPLDNREW EFQKYGH UniProtKB: E3 ubiquitin-protein ligase RBX1 |
-Macromolecule #3: Cullin-associated NEDD8-dissociated protein 1
| Macromolecule | Name: Cullin-associated NEDD8-dissociated protein 1 / type: protein_or_peptide / ID: 3 / Details: CAND1 b-hairpin++ (with M1068W P1070Q mutant) / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137.472297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSPEFPGRMA SASYHISNLL EKMTSSDKDF RFMATNDLMT ELQKDSIKLD DDSERKVVKM ILKLLEDKNG EVQNLAVKCL GPLVSKVKE YQVETIVDTL CTNMLSDKEQ LRDISSIGLK TVIGELPPAS SGSALAANVC KKITGRLTSA IAKQEDVSVQ L EALDIMAD ...String: GSPEFPGRMA SASYHISNLL EKMTSSDKDF RFMATNDLMT ELQKDSIKLD DDSERKVVKM ILKLLEDKNG EVQNLAVKCL GPLVSKVKE YQVETIVDTL CTNMLSDKEQ LRDISSIGLK TVIGELPPAS SGSALAANVC KKITGRLTSA IAKQEDVSVQ L EALDIMAD MLSRQGGLLV NFHPSILTCL LPQLTSPRLA VRKRTIIALG HLVMSCGNIV FVDLIEHLLS ELSKNDSMST TR TYIQCIA AISRQAGHRI GEYLEKIIPL VVKFCNVDDD ELREYCIQAF ESFVRRCPKE VYPHVSTIIN ICLKYLTYDP NYN YDDEDE DENAMDADGG DDDDQGSDDE YSDDDDMSWK VRRAAAKCLD AVVSTRHEML PEFYKTVSPA LISRFKEREE NVKA DVFHA YLSLLKQTRP VQSWLCDPDA MEQGETPLTM LQSQVPNIVK ALHKQMKEKS VKTRQCCFNM LTELVNVLPG ALTQH IPVL VPGIIFSLND KSSSSNLKID ALSCLYVILC NHSPQVFHPH VQALVPPVVA CVGDPFYKIT SEALLVTQQL VKVIRP LDQ PSSFDATPYI KDLFTCTIKR LKAADIDQEV KERAISCMGQ IICNLGDNLG SDLPNTLQIF LERLKNEITR LTTVKAL TL IAGSPLKIDL RPVLGEGVPI LASFLRKNQR ALKLGTLSAL DILIKNYSDS LTAAMIDAVL DELPPLISES DMHVSQMA I SFLTTLAKVY PSSLSKISGS ILNELIGLVR SPLLQGGALS AMLDFFQALV VTGTNNLGYM DLLRMLTGPV YSQSTALTH KQSYYSIAKC VAALTRACPK EGPAVVGQFI QDVKNSRSTD SIRLLALLSL GEVGHHIDLS GQLELKSVIL EAFSSPSEEV KSAASYALG SISVGNLPEY LPFVLQEITS QPKRQYLLLH SLKEIISSAS VVGLKPYVEN IWALLLKHCE CAEEGTRNVV V ECLGKLTL IDPETLLPRL KGYLISGSSY ARSSVVTAVK FTISDHPQPI DPLLKNCIGD FLKTLEDPDL NVRRVALVTF NS AAHNKPS LIRDLLDTVL PHLYNETKVR KELIREVEWG QFKHTVDDGL DIRKAAFECM YTLLDSCLDR LDIFEFLNHV EDG LKDHYD IKMLTFLMLV RLSTLCPSAV LQRLDRLVEP LRATCTTKVK ANSVKQEFEK QDELKRSAMR AVAALLTIPE AEKS PLMSE FQSQISSNPE LAAIFESIQK DSSSTNLESM DTS UniProtKB: Cullin-associated NEDD8-dissociated protein 1 |
-Macromolecule #4: S-phase kinase-associated protein 2
| Macromolecule | Name: S-phase kinase-associated protein 2 / type: protein_or_peptide / ID: 4 Details: SKP2 N-terminal region not visible in EM density. The LRR region of SKP2 was wholesale docked from previous structure with all sidechains removed. Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.96191 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSMHRKHLQE IPDLSSNVAT SFTWGWDSSK TSELLSGMGV SALEKEEPDS ENIPQELLSN LGHPESPPRK RLKSKGSDKD FVIVRRPKL NRENFPGVSW DSLPDELLLG IFSCLCLPEL LKVSGVCKRW YRLASDESLW QTLDLTGKNL HPDVTGRLLS Q GVIAFRCP ...String: GSMHRKHLQE IPDLSSNVAT SFTWGWDSSK TSELLSGMGV SALEKEEPDS ENIPQELLSN LGHPESPPRK RLKSKGSDKD FVIVRRPKL NRENFPGVSW DSLPDELLLG IFSCLCLPEL LKVSGVCKRW YRLASDESLW QTLDLTGKNL HPDVTGRLLS Q GVIAFRCP RSFMDQPLAE HFSPFRVQHM DLSNSVIEVS TLHGILSQCS KLQNLSLEGL RLSDPIVNTL AKNSNLVRLN LS GCSGFSE FALQTLLSSC SRLDELNLSW CFDFTEKHVQ VAVAHVSETI TQLNLSGYRK NLQKSDLSTL VRRCPNLVHL DLS DSVMLK NDCFQEFFQL NYLQHLSLSR CYDIIPETLL ELGEIPTLKT LQVFGIVPDG TLQLLKEALP HLQINCSHFT TIAR PTIGN KKNQEIWGIK CRLTLQKPSC L UniProtKB: S-phase kinase-associated protein 2 |
-Macromolecule #5: S-phase kinase-associated protein 1
| Macromolecule | Name: S-phase kinase-associated protein 1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.679965 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPSIKLQSSD GEIFEVDVEI AKQSVTIKTM LEDLGMDDEG DDDPVPLPNV NAAILKKVIQ WCTHHKDDPP PPEDDENKEK RTDDIPVWD QEFLKVDQGT LFELILAANY LDIKGLLDVT CKTVANMIKG KTPEEIRKTF NIKNDFTEEE EAQVRKENQW C EEK UniProtKB: S-phase kinase-associated protein 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 68.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)