+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CAND1-CUL1-RBX1 | |||||||||
 Map data Map data | CAND1-CUL1-RBX1 RELION postprocess | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cullin-RING E3 ubiquitin ligase / Assembly factor / CAND1 / LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationSCF complex assembly / negative regulation of catalytic activity / Parkin-FBXW7-Cul1 ubiquitin ligase complex / negative regulation of beige fat cell differentiation / cullin-RING-type E3 NEDD8 transferase / NEDD8 transferase activity / cullin-RING ubiquitin ligase complex / Cul7-RING ubiquitin ligase complex / cellular response to chemical stress / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling ...SCF complex assembly / negative regulation of catalytic activity / Parkin-FBXW7-Cul1 ubiquitin ligase complex / negative regulation of beige fat cell differentiation / cullin-RING-type E3 NEDD8 transferase / NEDD8 transferase activity / cullin-RING ubiquitin ligase complex / Cul7-RING ubiquitin ligase complex / cellular response to chemical stress / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / protein K27-linked ubiquitination / positive regulation of protein autoubiquitination / RNA polymerase II transcription initiation surveillance / protein neddylation / NEDD8 ligase activity / VCB complex / negative regulation of response to oxidative stress / Cul5-RING ubiquitin ligase complex / ubiquitin-ubiquitin ligase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / SCF ubiquitin ligase complex / Cul2-RING ubiquitin ligase complex / negative regulation of type I interferon production / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / Cul3-RING ubiquitin ligase complex / negative regulation of mitophagy / Cul4A-RING E3 ubiquitin ligase complex / Cul4-RING E3 ubiquitin ligase complex / Prolactin receptor signaling / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / cullin family protein binding / positive regulation of RNA polymerase II transcription preinitiation complex assembly / protein monoubiquitination / ubiquitin ligase complex / site of DNA damage / protein K48-linked ubiquitination / signal transduction in response to DNA damage / Nuclear events stimulated by ALK signaling in cancer / transcription-coupled nucleotide-excision repair / positive regulation of TORC1 signaling / regulation of cellular response to insulin stimulus / negative regulation of insulin receptor signaling pathway / intrinsic apoptotic signaling pathway / post-translational protein modification / TBP-class protein binding / T cell activation / negative regulation of canonical NF-kappaB signal transduction / animal organ morphogenesis / Regulation of BACH1 activity / MAP3K8 (TPL2)-dependent MAPK1/3 activation / Degradation of CRY and PER proteins / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / cellular response to amino acid stimulus / Degradation of DVL / Dectin-1 mediated noncanonical NF-kB signaling / Activation of NF-kappaB in B cells / G1/S transition of mitotic cell cycle / Degradation of GLI1 by the proteasome / negative regulation of canonical Wnt signaling pathway / Iron uptake and transport / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Negative regulation of NOTCH4 signaling / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / Hedgehog 'on' state / Recognition of DNA damage by PCNA-containing replication complex / Vif-mediated degradation of APOBEC3G / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / DNA Damage Recognition in GG-NER / Evasion by RSV of host interferon responses / NOTCH1 Intracellular Domain Regulates Transcription / CLEC7A (Dectin-1) signaling / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / SCF(Skp2)-mediated degradation of p27/p21 / Dual Incision in GG-NER / FCERI mediated NF-kB activation / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / Regulation of expression of SLITs and ROBOs / Formation of Incision Complex in GG-NER / Interleukin-1 signaling / protein polyubiquitination / Orc1 removal from chromatin / Regulation of RAS by GAPs / Dual incision in TC-NER / Regulation of RUNX2 expression and activity / Gap-filling DNA repair synthesis and ligation in TC-NER / Cyclin D associated events in G1 / positive regulation of protein catabolic process / ubiquitin-protein transferase activity / cellular response to UV / ubiquitin protein ligase activity / KEAP1-NFE2L2 pathway Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
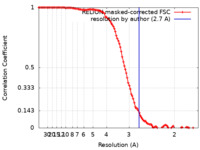
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Baek K / Schulman BA | |||||||||
| Funding support |  Germany, European Union, 2 items Germany, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Systemwide disassembly and assembly of SCF ubiquitin ligase complexes. Authors: Kheewoong Baek / Daniel C Scott / Lukas T Henneberg / Moeko T King / Matthias Mann / Brenda A Schulman /   Abstract: Cells respond to environmental cues by remodeling their inventories of multiprotein complexes. Cellular repertoires of SCF (SKP1-CUL1-F box protein) ubiquitin ligase complexes, which mediate much ...Cells respond to environmental cues by remodeling their inventories of multiprotein complexes. Cellular repertoires of SCF (SKP1-CUL1-F box protein) ubiquitin ligase complexes, which mediate much protein degradation, require CAND1 to distribute the limiting CUL1 subunit across the family of ∼70 different F box proteins. Yet, how a single factor coordinately assembles numerous distinct multiprotein complexes remains unknown. We obtained cryo-EM structures of CAND1-bound SCF complexes in multiple states and correlated mutational effects on structures, biochemistry, and cellular assays. The data suggest that CAND1 clasps idling catalytic domains of an inactive SCF, rolls around, and allosterically rocks and destabilizes the SCF. New SCF production proceeds in reverse, through SKP1-F box allosterically destabilizing CAND1. The CAND1-SCF conformational ensemble recycles CUL1 from inactive complexes, fueling mixing and matching of SCF parts for E3 activation in response to substrate availability. Our data reveal biogenesis of a predominant family of E3 ligases, and the molecular basis for systemwide multiprotein complex assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14561.map.gz emd_14561.map.gz | 11.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14561-v30.xml emd-14561-v30.xml emd-14561.xml emd-14561.xml | 25.7 KB 25.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14561_fsc.xml emd_14561_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_14561.png emd_14561.png | 118 KB | ||
| Masks |  emd_14561_msk_1.map emd_14561_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-14561.cif.gz emd-14561.cif.gz | 7.7 KB | ||
| Others |  emd_14561_additional_1.map.gz emd_14561_additional_1.map.gz emd_14561_additional_2.map.gz emd_14561_additional_2.map.gz emd_14561_half_map_1.map.gz emd_14561_half_map_1.map.gz emd_14561_half_map_2.map.gz emd_14561_half_map_2.map.gz | 98.5 MB 111.6 MB 98.6 MB 98.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14561 http://ftp.pdbj.org/pub/emdb/structures/EMD-14561 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14561 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14561 | HTTPS FTP |
-Related structure data
| Related structure data |  7z8rMC  7z8tC  7z8vC  7zbwC  7zbzC  8cdjC  8cdkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14561.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14561.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAND1-CUL1-RBX1 RELION postprocess | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8512 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_14561_msk_1.map emd_14561_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
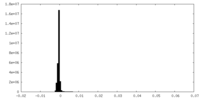
| Density Histograms |
-Additional map: CAND1-CUL1-RBX1 3D Refinement
| File | emd_14561_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAND1-CUL1-RBX1 3D Refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
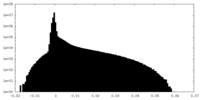
| Density Histograms |
-Additional map: CAND1-CUL1-RBX1 deepEMhancer
| File | emd_14561_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAND1-CUL1-RBX1 deepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
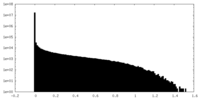
| Density Histograms |
-Half map: CAND1-CUL1-RBX1 halfmap1
| File | emd_14561_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAND1-CUL1-RBX1 halfmap1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
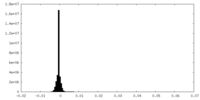
| Density Histograms |
-Half map: CAND1-CUL1-RBX1 halfmap2
| File | emd_14561_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CAND1-CUL1-RBX1 halfmap2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CAND1-CUL1-RBX1
| Entire | Name: CAND1-CUL1-RBX1 |
|---|---|
| Components |
|
-Supramolecule #1: CAND1-CUL1-RBX1
| Supramolecule | Name: CAND1-CUL1-RBX1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Cullin-1
| Macromolecule | Name: Cullin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 89.800367 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSSTRSQNPH GLKQIGLDQI WDDLRAGIQQ VYTRQSMAKS RYMELYTHVY NYCTSVHQSN QARGAGVPPS KSKKGQTPGG AQFVGLELY KRLKEFLKNY LTNLLKDGED LMDESVLKFY TQQWEDYRFS SKVLNGICAY LNRHWVRREC DEGRKGIYEI Y SLALVTWR ...String: MSSTRSQNPH GLKQIGLDQI WDDLRAGIQQ VYTRQSMAKS RYMELYTHVY NYCTSVHQSN QARGAGVPPS KSKKGQTPGG AQFVGLELY KRLKEFLKNY LTNLLKDGED LMDESVLKFY TQQWEDYRFS SKVLNGICAY LNRHWVRREC DEGRKGIYEI Y SLALVTWR DCLFRPLNKQ VTNAVLKLIE KERNGETINT RLISGVVQSY VELGLNEDDA FAKGPTLTVY KESFESQFLA DT ERFYTRE STEFLQQNPV TEYMKKAEAR LLEEQRRVQV YLHESTQDEL ARKCEQVLIE KHLEIFHTEF QNLLDADKNE DLG RMYNLV SRIQDGLGEL KKLLETHIHN QGLAAIEKCG EAALNDPKMY VQTVLDVHKK YNALVMSAFN NDAGFVAALD KACG RFINN NAVTKMAQSS SKSPELLARY CDSLLKKSSK NPEEAELEDT LNQVMVVFKY IEDKDVFQKF YAKMLAKRLV HQNSA SDDA EASMISKLKQ ACGFEYTSKL QRMFQDIGVS KDLNEQFKKH LTNSEPLDLD FSIQVLSSGS WPFQQSCTFA LPSELE RSY QRFTAFYASR HSGRKLTWLY QLSKGELVTN CFKNRYTLQA STFQMAILLQ YNTEDAYTVQ QLTDSTQIKM DILAQVL QI LLKSKLLVLE DENANVDEVE LKPDTLIKLY LGYKNKKLRV NINVPMKTEQ KQEQETTHKN IEEDRKLLIQ AAIVRIMK M RKVLKHQQLL GEVLTQLSSR FKPRVPVIKK CIDILIEKEY LERVDGEKDT YSYLA UniProtKB: Cullin-1 |
-Macromolecule #2: Cullin-associated NEDD8-dissociated protein 1
| Macromolecule | Name: Cullin-associated NEDD8-dissociated protein 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137.386281 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSPEFPGRMA SASYHISNLL EKMTSSDKDF RFMATNDLMT ELQKDSIKLD DDSERKVVKM ILKLLEDKNG EVQNLAVKCL GPLVSKVKE YQVETIVDTL CTNMLSDKEQ LRDISSIGLK TVIGELPPAS SGSALAANVC KKITGRLTSA IAKQEDVSVQ L EALDIMAD ...String: GSPEFPGRMA SASYHISNLL EKMTSSDKDF RFMATNDLMT ELQKDSIKLD DDSERKVVKM ILKLLEDKNG EVQNLAVKCL GPLVSKVKE YQVETIVDTL CTNMLSDKEQ LRDISSIGLK TVIGELPPAS SGSALAANVC KKITGRLTSA IAKQEDVSVQ L EALDIMAD MLSRQGGLLV NFHPSILTCL LPQLTSPRLA VRKRTIIALG HLVMSCGNIV FVDLIEHLLS ELSKNDSMST TR TYIQCIA AISRQAGHRI GEYLEKIIPL VVKFCNVDDD ELREYCIQAF ESFVRRCPKE VYPHVSTIIN ICLKYLTYDP NYN YDDEDE DENAMDADGG DDDDQGSDDE YSDDDDMSWK VRRAAAKCLD AVVSTRHEML PEFYKTVSPA LISRFKEREE NVKA DVFHA YLSLLKQTRP VQSWLCDPDA MEQGETPLTM LQSQVPNIVK ALHKQMKEKS VKTRQCCFNM LTELVNVLPG ALTQH IPVL VPGIIFSLND KSSSSNLKID ALSCLYVILC NHSPQVFHPH VQALVPPVVA CVGDPFYKIT SEALLVTQQL VKVIRP LDQ PSSFDATPYI KDLFTCTIKR LKAADIDQEV KERAISCMGQ IICNLGDNLG SDLPNTLQIF LERLKNEITR LTTVKAL TL IAGSPLKIDL RPVLGEGVPI LASFLRKNQR ALKLGTLSAL DILIKNYSDS LTAAMIDAVL DELPPLISES DMHVSQMA I SFLTTLAKVY PSSLSKISGS ILNELIGLVR SPLLQGGALS AMLDFFQALV VTGTNNLGYM DLLRMLTGPV YSQSTALTH KQSYYSIAKC VAALTRACPK EGPAVVGQFI QDVKNSRSTD SIRLLALLSL GEVGHHIDLS GQLELKSVIL EAFSSPSEEV KSAASYALG SISVGNLPEY LPFVLQEITS QPKRQYLLLH SLKEIISSAS VVGLKPYVEN IWALLLKHCE CAEEGTRNVV V ECLGKLTL IDPETLLPRL KGYLISGSSY ARSSVVTAVK FTISDHPQPI DPLLKNCIGD FLKTLEDPDL NVRRVALVTF NS AAHNKPS LIRDLLDTVL PHLYNETKVR KELIREVEMG PFKHTVDDGL DIRKAAFECM YTLLDSCLDR LDIFEFLNHV EDG LKDHYD IKMLTFLMLV RLSTLCPSAV LQRLDRLVEP LRATCTTKVK ANSVKQEFEK QDELKRSAMR AVAALLTIPE AEKS PLMSE FQSQISSNPE LAAIFESIQK DSSSTNLESM DTS UniProtKB: Cullin-associated NEDD8-dissociated protein 1 |
-Macromolecule #3: E3 ubiquitin-protein ligase RBX1, N-terminally processed
| Macromolecule | Name: E3 ubiquitin-protein ligase RBX1, N-terminally processed type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.089677 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GSMDVDTPSG TNSGAGKKRF EVKKWNAVAL WAWDIVVDNC AICRNHIMDL CIECQANQAS ATSEECTVAW GVCNHAFHFH CISRWLKTR QVCPLDNREW EFQKYGH UniProtKB: E3 ubiquitin-protein ligase RBX1 |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 3 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



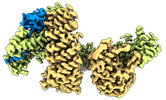





















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)