[English] 日本語
 Yorodumi
Yorodumi- EMDB-16549: structure of LEDGF/p75 PWWP domain bound to the H3K36 trimethylat... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | structure of LEDGF/p75 PWWP domain bound to the H3K36 trimethylated dinucleosome | ||||||||||||
 Map data Map data | LAFTER filtered | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | nucleosome / transcription activator / methylation / complex / DNA BINDING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationIntegration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / supercoiled DNA binding / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Formation of WDR5-containing histone-modifying complexes / mRNA 5'-splice site recognition / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / heterochromatin ...Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / supercoiled DNA binding / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Formation of WDR5-containing histone-modifying complexes / mRNA 5'-splice site recognition / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / heterochromatin / nuclear periphery / euchromatin / structural constituent of chromatin / heterochromatin formation / nucleosome / nucleosome assembly / response to heat / response to oxidative stress / DNA-binding transcription factor binding / transcription coactivator activity / chromatin remodeling / protein heterodimerization activity / chromatin binding / positive regulation of transcription by RNA polymerase II / DNA binding / RNA binding / nucleoplasm / nucleus / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
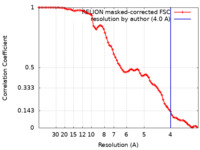
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | ||||||||||||
 Authors Authors | Koutna E / Kouba T / Novacek J / Veverka V | ||||||||||||
| Funding support |  Czech Republic, European Union, 3 items Czech Republic, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Multivalency of nucleosome recognition by LEDGF. Authors: Eliška Koutná / Vanda Lux / Tomáš Kouba / Jana Škerlová / Jiří Nováček / Pavel Srb / Rozálie Hexnerová / Hana Šváchová / Zdeněk Kukačka / Petr Novák / Milan Fábry / Simon ...Authors: Eliška Koutná / Vanda Lux / Tomáš Kouba / Jana Škerlová / Jiří Nováček / Pavel Srb / Rozálie Hexnerová / Hana Šváchová / Zdeněk Kukačka / Petr Novák / Milan Fábry / Simon Poepsel / Václav Veverka /   Abstract: Eukaryotic transcription is dependent on specific histone modifications. Their recognition by chromatin readers triggers complex processes relying on the coordinated association of transcription ...Eukaryotic transcription is dependent on specific histone modifications. Their recognition by chromatin readers triggers complex processes relying on the coordinated association of transcription regulatory factors. Although various modification states of a particular histone residue often lead to differential outcomes, it is not entirely clear how they are discriminated. Moreover, the contribution of intrinsically disordered regions outside of the specialized reader domains to nucleosome binding remains unexplored. Here, we report the structures of a PWWP domain from transcriptional coactivator LEDGF in complex with the H3K36 di- and trimethylated nucleosome, indicating that both methylation marks are recognized by PWWP in a highly conserved manner. We identify a unique secondary interaction site for the PWWP domain at the interface between the acidic patch and nucleosomal DNA that might contribute to an H3K36-methylation independent role of LEDGF. We reveal DNA interacting motifs in the intrinsically disordered region of LEDGF that discriminate between the intra- or extranucleosomal DNA but remain dynamic in the context of dinucleosomes. The interplay between the LEDGF H3K36-methylation reader and protein binding module mediated by multivalent interactions of the intrinsically disordered linker with chromatin might help direct the elongation machinery to the vicinity of RNA polymerase II, thereby facilitating productive elongation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16549.map.gz emd_16549.map.gz | 38.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16549-v30.xml emd-16549-v30.xml emd-16549.xml emd-16549.xml | 27.2 KB 27.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16549_fsc.xml emd_16549_fsc.xml | 7.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_16549.png emd_16549.png | 90.9 KB | ||
| Masks |  emd_16549_msk_1.map emd_16549_msk_1.map | 40.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16549.cif.gz emd-16549.cif.gz | 7.4 KB | ||
| Others |  emd_16549_additional_1.map.gz emd_16549_additional_1.map.gz emd_16549_half_map_1.map.gz emd_16549_half_map_1.map.gz emd_16549_half_map_2.map.gz emd_16549_half_map_2.map.gz | 38 MB 31.3 MB 31.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16549 http://ftp.pdbj.org/pub/emdb/structures/EMD-16549 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16549 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16549 | HTTPS FTP |
-Related structure data
| Related structure data |  8cbqMC  8cbnC  8pc5C  8pc6C  8peoC  8pepC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16549.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16549.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LAFTER filtered | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.656 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16549_msk_1.map emd_16549_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: RELION post-processed
| File | emd_16549_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RELION post-processed | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16549_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16549_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : H3K36me3 dinucleosome-LEDGF/p75 PWWP domain complex
+Supramolecule #1: H3K36me3 dinucleosome-LEDGF/p75 PWWP domain complex
+Supramolecule #2: Histone
+Supramolecule #3: DNA
+Supramolecule #4: LEDGF/p75 PWWP domain complex
+Macromolecule #1: Histone H4
+Macromolecule #2: Histone H2A
+Macromolecule #3: Histone H2B 1.1
+Macromolecule #6: PC4 and SFRS1-interacting protein
+Macromolecule #7: Histone H3
+Macromolecule #4: WIDOM 601 DNA
+Macromolecule #5: WIDOM 601 DNA
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.2 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)















































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)