[English] 日本語
 Yorodumi
Yorodumi- EMDB-16483: In vitro structure of the Nitrosopumilus maritimus S-layer - Two-... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In vitro structure of the Nitrosopumilus maritimus S-layer - Two-fold symmetry (C2) | |||||||||
 Map data Map data | PostProcessed map with B-factor sharpening | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Nmar_1547 S-layer / STRUCTURAL PROTEIN | |||||||||
| Function / homology | membrane / Uncharacterized protein Function and homology information Function and homology information | |||||||||
| Biological species |  Nitrosopumilus maritimus SCM1 (archaea) Nitrosopumilus maritimus SCM1 (archaea) | |||||||||
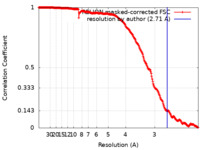
| Method | single particle reconstruction / cryo EM / Resolution: 2.71 Å | |||||||||
 Authors Authors | von Kuegelgen A / Bharat T | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Membraneless channels sieve cations in ammonia-oxidizing marine archaea. Authors: Andriko von Kügelgen / C Keith Cassidy / Sofie van Dorst / Lennart L Pagani / Christopher Batters / Zephyr Ford / Jan Löwe / Vikram Alva / Phillip J Stansfeld / Tanmay A M Bharat /    Abstract: Nitrosopumilus maritimus is an ammonia-oxidizing archaeon that is crucial to the global nitrogen cycle. A critical step for nitrogen oxidation is the entrapment of ammonium ions from a dilute marine ...Nitrosopumilus maritimus is an ammonia-oxidizing archaeon that is crucial to the global nitrogen cycle. A critical step for nitrogen oxidation is the entrapment of ammonium ions from a dilute marine environment at the cell surface and their subsequent channelling to the cell membrane of N. maritimus. Here we elucidate the structure of the molecular machinery responsible for this process, comprising the surface layer (S-layer), using electron cryotomography and subtomogram averaging from cells. We supplemented our in situ structure of the ammonium-binding S-layer array with a single-particle electron cryomicroscopy structure, revealing detailed features of this immunoglobulin-rich and glycan-decorated S-layer. Biochemical analyses showed strong ammonium binding by the cell surface, which was lost after S-layer disassembly. Sensitive bioinformatic analyses identified similar S-layers in many ammonia-oxidizing archaea, with conserved sequence and structural characteristics. Moreover, molecular simulations and structure determination of ammonium-enriched specimens enabled us to examine the cation-binding properties of the S-layer, revealing how it concentrates ammonium ions on its cell-facing side, effectively acting as a multichannel sieve on the cell membrane. This in situ structural study illuminates the biogeochemically essential process of ammonium binding and channelling, common to many marine microorganisms that are fundamental to the nitrogen cycle. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16483.map.gz emd_16483.map.gz | 114 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16483-v30.xml emd-16483-v30.xml emd-16483.xml emd-16483.xml | 26.8 KB 26.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16483_fsc.xml emd_16483_fsc.xml | 22.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_16483.png emd_16483.png | 290 KB | ||
| Masks |  emd_16483_msk_1.map emd_16483_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16483.cif.gz emd-16483.cif.gz | 8.6 KB | ||
| Others |  emd_16483_additional_1.map.gz emd_16483_additional_1.map.gz emd_16483_half_map_1.map.gz emd_16483_half_map_1.map.gz emd_16483_half_map_2.map.gz emd_16483_half_map_2.map.gz | 112.5 MB 113.7 MB 113.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16483 http://ftp.pdbj.org/pub/emdb/structures/EMD-16483 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16483 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16483 | HTTPS FTP |
-Validation report
| Summary document |  emd_16483_validation.pdf.gz emd_16483_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16483_full_validation.pdf.gz emd_16483_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_16483_validation.xml.gz emd_16483_validation.xml.gz | 22.4 KB | Display | |
| Data in CIF |  emd_16483_validation.cif.gz emd_16483_validation.cif.gz | 30.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16483 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16483 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16483 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16483 | HTTPS FTP |
-Related structure data
| Related structure data |  8c8lMC  8c8kC  8c8mC  8c8nC  8c8oC  8c8rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16483.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16483.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | PostProcessed map with B-factor sharpening | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.092 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16483_msk_1.map emd_16483_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Full map with out b-factor sharpening
| File | emd_16483_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map with out b-factor sharpening | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_16483_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_16483_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Nitrosopumilus maritimus S-layer
| Entire | Name: Nitrosopumilus maritimus S-layer |
|---|---|
| Components |
|
-Supramolecule #1: Nitrosopumilus maritimus S-layer
| Supramolecule | Name: Nitrosopumilus maritimus S-layer / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Nitrosopumilus maritimus S-layer C2 symmetrised |
|---|---|
| Source (natural) | Organism:  Nitrosopumilus maritimus SCM1 (archaea) / Strain: SCM1 / Location in cell: extracellular Nitrosopumilus maritimus SCM1 (archaea) / Strain: SCM1 / Location in cell: extracellular |
-Macromolecule #1: Cell surface protein
| Macromolecule | Name: Cell surface protein / type: protein_or_peptide / ID: 1 / Details: In-vitro isolated S-layer / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Nitrosopumilus maritimus SCM1 (archaea) / Strain: SCM1 Nitrosopumilus maritimus SCM1 (archaea) / Strain: SCM1 |
| Molecular weight | Theoretical: 183.156 KDa |
| Sequence | String: MNNEIGRKIT SLTLMTIMVA GGLTFAIPGV MPEAMAANAN LFVSAENSQF DNYMSGPQVI EVVVIDSDIN DTDEAKGEPD VTVNGKVLR MVQAVDGNWY GYFADRDQAQ IADSTATTAD SGLDFGVFCA SSSGTAALGF STTETDGIAI PITIANATAT G NGTQTGSS ...String: MNNEIGRKIT SLTLMTIMVA GGLTFAIPGV MPEAMAANAN LFVSAENSQF DNYMSGPQVI EVVVIDSDIN DTDEAKGEPD VTVNGKVLR MVQAVDGNWY GYFADRDQAQ IADSTATTAD SGLDFGVFCA SSSGTAALGF STTETDGIAI PITIANATAT G NGTQTGSS SGGAITTTCA ANTLDASTAN GTINVVREAK DPVAASGSVS VGQIGLKNGT ANSGPNWPFI QLYELNPTGN VV VQYNKGG GVQSTTLTFD TVDQFAELSL DRTVFPRVSQ VHATITDLWL NIDPTDEDSW TFATNTKNTT SSFNVDTFYQ VFD ENGASG GSALTLRTTL SSLMCEDNCV LTLDVDAQSS GTPVVTIQDN GDSILTQLNA SSNTNANNAS AFGISTETAK LGTG SIPVT ITEQGPNSGV FGTYDESDKS VLKITDNAKR GTSASLDYNE TPQTILVGFS FASIDIQPVT DEWTSGQEIP VVIVD ADQN KNSRADEDLD LNNPDVTLIP ALRTGDPFTI DEGGTPSLIF TNGTNGDDSI FDTGAINNTS AGQVGNFTLN INVTRF SSA TNITSTESID TFSKRLISAQ TANSSANFDV DFAIIDLGSA TLETLKETVV DEDNTAVGFN FFNYDVRSLG ADTVSIA LL NTTGNILPWV NNDTRNVDKN NAILLVSNST NSQAYVDLTN AVSDAVYGST NTDSNVNIGF AMYFTGVGDL AAKEVIVM D FFSFGFTDDG VQSSERFANQ IIRIEAEETG DNTSTFEGSL EYVMVNQINI QDAGTFSGIT PIADDPSFIV IEDLTDEDA PRVNYNDLGA DGVTTPVSDQ EEAPSHSGVV SLNADSYKIA DTVVITVEDL DLNVDSDLID IFTVVSDNSK ATDDAVGSAT TQSLSFGEL GRLLDVTFDD VIWSTPDGAN NTATGNDSDT CSTELSNAGI TDTGLGATGF TLVETGAATG VFVGDFQIPS F WCRVSDTT TTPYTYAGDE ETTTGLDIEV NYVDFRDASG EIVEVGDSAG VRANTGSVSL DRTVYPVPFG TIADSSKAAN AA PNGRSVF PIHATGITST IDSTEELPTG DLTIHVRIND PDFDENPAGE DAMDQDNALK ISVIRGSDSV VLGYAGASER TGK IDVGGN NGTISNIRSF GEMDEIAPDA GIFELDVNIK FTDGPASAQC NSHDTLYTAL DGTTGKADTN RFDDGAASGQ EYCI LQGDI LQVEYTDPAD ASGDANTVTD SATFDLRNGV LQSDKSVYII GSDMILTLIE PDFDLDNDSA ETYDLDLIEW DSDAA TTTM GNKGVTGAAA AFDPEPTDFR ETGDSTGIFQ IVIEIPESLS NDKLERGEEI ILEYTDWGPS GSDYVGDEDE DVNLTI YTS NFGATVELDQ KVYSWTDKVY ITIVAPDHNF DSDLVDEIGE TDSDPIKVST RGFDLDNYKL VETGTDTGIF TGEVILT GF TAHDADGDGN TGDATGTTSG SGPTDGLLAT DDDDGLTVSF EFSEDETIVG SALIRWNIGE VQWLEASYPA SGTGVVRV I DPDMNLDPEA VDNFEVDVWS DSDAGGIDLT VTETNEATGI FEGTVFFTTL DESSGHRLRV SEGDTVTAEY EDNTLPDPY TTADELDITA TSLIGTVVPP LERAPAANLR TVDAFGNSLD SVSVDQQVQI SADLANGQDR EQSFAYLVQI QDANGVTVSL AWITGSLSS GQSFSPALSW IPTEAGTYTA TAFVWESVDN PTALSPPVST TVNVS UniProtKB: Uncharacterized protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 50 mM HEPES/NaOH pH=7.5, 500 mM NaCl, 50 mM MgCl2, 10 mM CaCl2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER/RHODIUM / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR / Details: 15 mA | |||||||||||||||
| Vitrification | Cryogen name: NITROGEN / Chamber humidity: 100 % / Chamber temperature: 283.15 K / Instrument: FEI VITROBOT MARK IV Details: absorption for 60 sec and blotted for 5 sec with blot force -10. | |||||||||||||||
| Details | In vitro isolate S-layer cell envelopes |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Specialist optics | Spherical aberration corrector: not used / Chromatic aberration corrector: not used / Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 3 / Number real images: 12557 / Average exposure time: 4.2 sec. / Average electron dose: 48.5 e/Å2 Details: collected over three sessions with one session with the stage tilted by 33 degrees (alpha tilt) |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 5.0 µm / Calibrated defocus min: 2.0 µm / Calibrated magnification: 81000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL / Overall B value: 41.4 / Target criteria: Best Fit |
|---|---|
| Output model |  PDB-8c8l: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)