[English] 日本語
 Yorodumi
Yorodumi- EMDB-1611: 7.5 Amstrong resolution cryo-electron microscopy reconstruction o... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
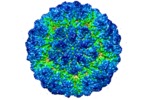
| Title | 7.5 Amstrong resolution cryo-electron microscopy reconstruction of Penicillium chrysogenum virus (PcV) | |||||||||
 Map data Map data | Density map of Penicillium chrysogenum virus (PcV) empty capsid | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | PcV / dsRNA viruses / T2 capsid / structural duplication / empty capsid | |||||||||
| Biological species |  Penicillium chrysogenum virus Penicillium chrysogenum virus | |||||||||
| Method | single particle reconstruction / cryo EM / negative staining / Resolution: 7.5 Å | |||||||||
 Authors Authors | Luque D / Gonzalez JM / Garriga D / Ghabrial SA / Trus B / Verdaguer N / Carrascosa JL / Caston JR | |||||||||
 Citation Citation |  Journal: J Virol / Year: 2010 Journal: J Virol / Year: 2010Title: The T=1 capsid protein of Penicillium chrysogenum virus is formed by a repeated helix-rich core indicative of gene duplication. Authors: Daniel Luque / José M González / Damiá Garriga / Said A Ghabrial / Wendy M Havens / Benes Trus / Nuria Verdaguer / José L Carrascosa / José R Castón /  Abstract: Penicillium chrysogenum virus (PcV), a member of the Chrysoviridae family, is a double-stranded RNA (dsRNA) fungal virus with a multipartite genome, with each RNA molecule encapsidated in a separate ...Penicillium chrysogenum virus (PcV), a member of the Chrysoviridae family, is a double-stranded RNA (dsRNA) fungal virus with a multipartite genome, with each RNA molecule encapsidated in a separate particle. Chrysoviruses lack an extracellular route and are transmitted during sporogenesis and cell fusion. The PcV capsid, based on a T=1 lattice containing 60 subunits of the 982-amino-acid capsid protein, remains structurally undisturbed throughout the viral cycle, participates in genome metabolism, and isolates the virus genome from host defense mechanisms. Using three-dimensional cryoelectron microscopy, we determined the structure of the PcV virion at 8.0 A resolution. The capsid protein has a high content of rod-like densities characteristic of alpha-helices, forming a repeated alpha-helical core indicative of gene duplication. Whereas the PcV capsid protein has two motifs with the same fold, most dsRNA virus capsid subunits consist of dimers of a single protein with similar folds. The spatial arrangement of the alpha-helical core resembles that found in the capsid protein of the L-A virus, a fungal totivirus with an undivided genome, suggesting a conserved basic fold. The encapsidated genome is organized in concentric shells; whereas the inner dsRNA shells are well defined, the outermost layer is dense due to numerous interactions with the inner capsid surface, specifically, six interacting areas per monomer. The outermost genome layer is arranged in an icosahedral cage, sufficiently well ordered to allow for modeling of an A-form dsRNA. The genome ordering might constitute a framework for dsRNA transcription at the capsid interior and/or have a structural role for capsid stability. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1611.map.gz emd_1611.map.gz | 140.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1611-v30.xml emd-1611-v30.xml emd-1611.xml emd-1611.xml | 8.3 KB 8.3 KB | Display Display |  EMDB header EMDB header |
| Images |  1611.gif 1611.gif EMD-1611.png EMD-1611.png | 119.1 KB 512.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1611 http://ftp.pdbj.org/pub/emdb/structures/EMD-1611 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1611 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1611 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1611.map.gz / Format: CCP4 / Size: 147.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1611.map.gz / Format: CCP4 / Size: 147.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density map of Penicillium chrysogenum virus (PcV) empty capsid | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Penicillium chrysogenum virus (PcV) empty particles
| Entire | Name: Penicillium chrysogenum virus (PcV) empty particles |
|---|---|
| Components |
|
-Supramolecule #1000: Penicillium chrysogenum virus (PcV) empty particles
| Supramolecule | Name: Penicillium chrysogenum virus (PcV) empty particles / type: sample / ID: 1000 / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 6.5 MDa |
-Supramolecule #1: Penicillium chrysogenum virus
| Supramolecule | Name: Penicillium chrysogenum virus / type: virus / ID: 1 / Name.synonym: PcV / NCBI-ID: 158372 / Sci species name: Penicillium chrysogenum virus / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: Yes / Syn species name: PcV |
|---|---|
| Host (natural) | Organism:  Penicillium chrysogenum (fungus) / synonym: FUNGI Penicillium chrysogenum (fungus) / synonym: FUNGI |
| Virus shell | Shell ID: 1 / Name: CP / Diameter: 400 Å / T number (triangulation number): 1 |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 / Details: 50 mM Tris-HCl pH 7.8, 5 mM EDTA,150 mM NaCl |
|---|---|
| Staining | Type: NEGATIVE Details: Samples of empty- and full-enriched particles fractions were applied to one side of a holey carbon grid, blotted and plunged into liquid ethane |
| Vitrification | Cryogen name: ETHANE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Image recording | Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 56 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: Each particle |
|---|---|
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Resolution.type: BY AUTHOR / Resolution: 7.5 Å / Resolution method: FSC 0.33 CUT-OFF / Software - Name: PFT2,EM3DR2 / Number images used: 5692 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















