[English] 日本語
 Yorodumi
Yorodumi- EMDB-16101: Cryo-EM structure of a contractile injection system in Streptomyc... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a contractile injection system in Streptomyces coelicolor, the sheath-tube module in extended state. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Structural protein / Contractile injection system | ||||||||||||
| Function / homology |  Function and homology information Function and homology information | ||||||||||||
| Biological species |  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) | ||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.9 Å | ||||||||||||
 Authors Authors | Casu B / Sallmen JW / Schlimpert S / Pilhofer M | ||||||||||||
| Funding support |  Switzerland, European Union, Switzerland, European Union,  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2023 Journal: Nat Microbiol / Year: 2023Title: Cytoplasmic contractile injection systems mediate cell death in Streptomyces. Authors: Bastien Casu / Joseph W Sallmen / Susan Schlimpert / Martin Pilhofer /   Abstract: Contractile injection systems (CIS) are bacteriophage tail-like structures that mediate bacterial cell-cell interactions. While CIS are highly abundant across diverse bacterial phyla, representative ...Contractile injection systems (CIS) are bacteriophage tail-like structures that mediate bacterial cell-cell interactions. While CIS are highly abundant across diverse bacterial phyla, representative gene clusters in Gram-positive organisms remain poorly studied. Here we characterize a CIS in the Gram-positive multicellular model organism Streptomyces coelicolor and show that, in contrast to most other CIS, S. coelicolor CIS (CIS) mediate cell death in response to stress and impact cellular development. CIS are expressed in the cytoplasm of vegetative hyphae and are not released into the medium. Our cryo-electron microscopy structure enabled the engineering of non-contractile and fluorescently tagged CIS assemblies. Cryo-electron tomography showed that CIS contraction is linked to reduced cellular integrity. Fluorescence light microscopy furthermore revealed that functional CIS mediate cell death upon encountering different types of stress. The absence of functional CIS had an impact on hyphal differentiation and secondary metabolite production. Finally, we identified three putative effector proteins, which when absent, phenocopied other CIS mutants. Our results provide new functional insights into CIS in Gram-positive organisms and a framework for studying novel intracellular roles, including regulated cell death and life-cycle progression in multicellular bacteria. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16101.map.gz emd_16101.map.gz | 117.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16101-v30.xml emd-16101-v30.xml emd-16101.xml emd-16101.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16101.png emd_16101.png | 46.3 KB | ||
| Filedesc metadata |  emd-16101.cif.gz emd-16101.cif.gz | 5.4 KB | ||
| Others |  emd_16101_half_map_1.map.gz emd_16101_half_map_1.map.gz emd_16101_half_map_2.map.gz emd_16101_half_map_2.map.gz | 96.9 MB 97 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16101 http://ftp.pdbj.org/pub/emdb/structures/EMD-16101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16101 | HTTPS FTP |
-Related structure data
| Related structure data |  8bl4MC  8bkyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16101.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16101.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_16101_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
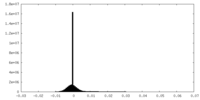
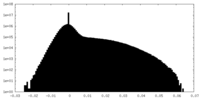
| Density Histograms |
-Half map: #1
| File | emd_16101_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
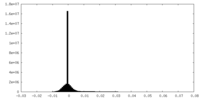
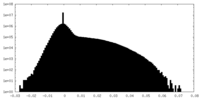
| Density Histograms |
- Sample components
Sample components
-Entire : The sheath-tube module of a contractile injection system in Strep...
| Entire | Name: The sheath-tube module of a contractile injection system in Streptomyces coelicolor |
|---|---|
| Components |
|
-Supramolecule #1: The sheath-tube module of a contractile injection system in Strep...
| Supramolecule | Name: The sheath-tube module of a contractile injection system in Streptomyces coelicolor type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) |
-Macromolecule #1: Phage tail sheath family protein
| Macromolecule | Name: Phage tail sheath family protein / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) |
| Molecular weight | Theoretical: 57.465113 KDa |
| Sequence | String: MPSYLSPGVY VEEVASGSRP IEGVGIEGVG TSVAAFVGLA PTGPLNEPTL VTNWTQYVAA FGDFTGGYYL AHSVYGFFNN GGSAAYVVR VGGSAEDAAA DGSVNGAAAP AAVTGSTAKA LPAAEPKQLG TFAVTATAAG QSGPLTVEVA DPEGEGPAER F KLIVKDGD ...String: MPSYLSPGVY VEEVASGSRP IEGVGIEGVG TSVAAFVGLA PTGPLNEPTL VTNWTQYVAA FGDFTGGYYL AHSVYGFFNN GGSAAYVVR VGGSAEDAAA DGSVNGAAAP AAVTGSTAKA LPAAEPKQLG TFAVTATAAG QSGPLTVEVA DPEGEGPAER F KLIVKDGD KPVETFDVSA KKGNRSYVVT QVKERSKLIT VTEAAPSAQL VRPENQSLTL PAPPSAAPAV PAGQAESAHP GP AQYLGDS SDRTGFGGLE AIDEISMVAV PDLMAAYQRG AIDLEAVKAV QLGLIAHCEL MGDRVAIIDP PPNQNARQIR VWR QETAGY DSKYAALYYP WIKSFDPATG QSRLVPPSGH VAGIWARNDS ERGVHKAPAN EVVRGAVDLE LQITRGEQDL LNPI GVNCI RSFPGRGIRV WGARTLSSDP AWRYLNIRRY FNYLEESILI GTQWVVFEPN DHNLWARIRR NVSAFLVNEW RNGAL FGQS PDQAYYVKCD EETNPPESVD LGRVVCEIGI APVKPAEFVI FRLAQFSSGG GELDE UniProtKB: Phage tail sheath family protein |
-Macromolecule #2: Phage tail protein
| Macromolecule | Name: Phage tail protein / type: protein_or_peptide / ID: 2 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Streptomyces coelicolor A3(2) (bacteria) Streptomyces coelicolor A3(2) (bacteria) |
| Molecular weight | Theoretical: 16.493668 KDa |
| Sequence | String: MSLPKPEDVL VAPNFGIQID GVMVEYLNSV SNLQIEQDVI RYQQNQGTTG RNNVTLMPGV AKDGSVQVER GMSQSSVFTQ WINDSMAGR MATARKNATI IVMDYEDNPV KRWNLRNAWC SKVVAGTLKA GDTNALTETI TIVFEELVVE UniProtKB: Phage tail protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 38.5 Å Applied symmetry - Helical parameters - Δ&Phi: 23.10 ° Applied symmetry - Helical parameters - Axial symmetry: C6 (6 fold cyclic) Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 18822 |
|---|---|
| Startup model | Type of model: EMDB MAP EMDB ID: |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)