+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GABA-A receptor a5 homomer - a5V3 - RO7172670 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | pLGIC GABA Neurotransmission / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationGABA receptor binding / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation / postsynaptic specialization membrane / neuronal cell body membrane ...GABA receptor binding / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation / postsynaptic specialization membrane / neuronal cell body membrane / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / cochlea development / associative learning / chloride channel complex / behavioral fear response / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / chloride transmembrane transport / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / signaling receptor activity / presynaptic membrane / postsynapse / signal transduction / nucleoplasm / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
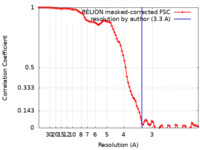
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Miller PS / Malinauskas TM / Hardwick SW / Kasaragod VB | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: The molecular basis of drug selectivity for α5 subunit-containing GABA receptors. Authors: Vikram Babu Kasaragod / Tomas Malinauskas / Ayla A Wahid / Judith Lengyel / Frederic Knoflach / Steven W Hardwick / Charlotte F Jones / Wan-Na Chen / Xavier Lucas / Kamel El Omari / Dimitri ...Authors: Vikram Babu Kasaragod / Tomas Malinauskas / Ayla A Wahid / Judith Lengyel / Frederic Knoflach / Steven W Hardwick / Charlotte F Jones / Wan-Na Chen / Xavier Lucas / Kamel El Omari / Dimitri Y Chirgadze / A Radu Aricescu / Giuseppe Cecere / Maria-Clemencia Hernandez / Paul S Miller /   Abstract: α5 subunit-containing γ-aminobutyric acid type A (GABA) receptors represent a promising drug target for neurological and neuropsychiatric disorders. Altered expression and function contributes to ...α5 subunit-containing γ-aminobutyric acid type A (GABA) receptors represent a promising drug target for neurological and neuropsychiatric disorders. Altered expression and function contributes to neurodevelopmental disorders such as Dup15q and Angelman syndromes, developmental epilepsy and autism. Effective drug action without side effects is dependent on both α5-subtype selectivity and the strength of the positive or negative allosteric modulation (PAM or NAM). Here we solve structures of drugs bound to the α5 subunit. These define the molecular basis of binding and α5 selectivity of the β-carboline, methyl 6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM), type II benzodiazepine NAMs, and a series of isoxazole NAMs and PAMs. For the isoxazole series, each molecule appears as an 'upper' and 'lower' moiety in the pocket. Structural data and radioligand binding data reveal a positional displacement of the upper moiety containing the isoxazole between the NAMs and PAMs. Using a hybrid molecule we directly measure the functional contribution of the upper moiety to NAM versus PAM activity. Overall, these structures provide a framework by which to understand distinct modulator binding modes and their basis of α5-subtype selectivity, appreciate structure-activity relationships, and empower future structure-based drug design campaigns. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16066.map.gz emd_16066.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16066-v30.xml emd-16066-v30.xml emd-16066.xml emd-16066.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16066_fsc.xml emd_16066_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_16066.png emd_16066.png | 173.9 KB | ||
| Filedesc metadata |  emd-16066.cif.gz emd-16066.cif.gz | 5.7 KB | ||
| Others |  emd_16066_half_map_1.map.gz emd_16066_half_map_1.map.gz emd_16066_half_map_2.map.gz emd_16066_half_map_2.map.gz | 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16066 http://ftp.pdbj.org/pub/emdb/structures/EMD-16066 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16066 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16066 | HTTPS FTP |
-Related structure data
| Related structure data |  8bhqMC  8bejC  8bgiC  8bhaC  8bhbC  8bhgC  8bhiC  8bhkC  8bhmC  8bhoC  8bhrC  8bhsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16066.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16066.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_16066_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
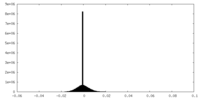
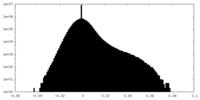
| Density Histograms |
-Half map: #2
| File | emd_16066_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
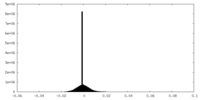
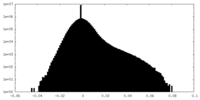
| Density Histograms |
- Sample components
Sample components
-Entire : GABA-A a5 subunit of homopentamer complex called a5V3
| Entire | Name: GABA-A a5 subunit of homopentamer complex called a5V3 |
|---|---|
| Components |
|
-Supramolecule #1: GABA-A a5 subunit of homopentamer complex called a5V3
| Supramolecule | Name: GABA-A a5 subunit of homopentamer complex called a5V3 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 205 KDa |
-Macromolecule #1: Gamma-aminobutyric acid receptor subunit alpha-5
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit alpha-5 / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.05791 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QMPTSSVKDE TNDNITIFTR ILDGLLDGYD NRLRPGLGER ITQVRTDMYV NSFGPVSDTE MEYTIDIFFA QTWKDERLRF KGPMQRLPL NNLLASKIWT PDTFFHNGKK SFAHWMTTPN RMLRIWNDGR VLYTLRLTIS AECPMDLEDF PMDEQNCPLK F GSYAYPNS ...String: QMPTSSVKDE TNDNITIFTR ILDGLLDGYD NRLRPGLGER ITQVRTDMYV NSFGPVSDTE MEYTIDIFFA QTWKDERLRF KGPMQRLPL NNLLASKIWT PDTFFHNGKK SFAHWMTTPN RMLRIWNDGR VLYTLRLTIS AECPMDLEDF PMDEQNCPLK F GSYAYPNS EVVYVWTNGS TKSVVVAEDG SRLNQYHLMG QTVGTENIST STGEYTIMTA HFHLKRKIGY FVIQTYLPCI MT VILSQVS FWLNRESVAA RTVFGVTTVL TMTTLSISAR NSLPKVAYAT AMDWFIAVCY AFVFSALLEF AFVNYITKSQ PAR AAKIDK MSRIVFPILF GTFNLVYWAT YLNGTTETSQ VAPA UniProtKB: Gamma-aminobutyric acid receptor subunit alpha-5, Gamma-aminobutyric acid receptor subunit alpha-5 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: 2-[[5-methyl-3-(6-methylpyridazin-3-yl)-1,2-oxazol-4-yl]methyl]-5...
| Macromolecule | Name: 2-[[5-methyl-3-(6-methylpyridazin-3-yl)-1,2-oxazol-4-yl]methyl]-5-(5-oxa-2-azaspiro[3.5]nonan-2-yl)pyridazin-3-one type: ligand / ID: 3 / Number of copies: 5 / Formula: QR3 |
|---|---|
| Molecular weight | Theoretical: 408.454 Da |
| Chemical component information | 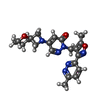 ChemComp-QR3: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DIFFRACTION / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.9 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)