+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human rhinovirus 2 empty particle in situ | |||||||||
 Map data Map data | EM map after pixel size scaling presented in 2-fold on Z (MRC standard) orientation. Micrographs were collected on lamellipodia of Cos7 cells infected with HRV2. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Human rhinovirus 2 / empty particle / in situ / cryo-EM. / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  rhinovirus A2 rhinovirus A2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.1 Å | |||||||||
 Authors Authors | Ishemgulova A / Mukhamedova L / Trebichalska Z / Payne P / Smerdova L / Moravcova J / Hrebik D / Buchta D / Skubnik K / Fuzik T ...Ishemgulova A / Mukhamedova L / Trebichalska Z / Payne P / Smerdova L / Moravcova J / Hrebik D / Buchta D / Skubnik K / Fuzik T / Novacek J / Plevka P | |||||||||
| Funding support |  Czech Republic, 1 items Czech Republic, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Endosome rupture enables enteroviruses to infect cells. Authors: Ishemgulova A | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15711.map.gz emd_15711.map.gz | 4.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15711-v30.xml emd-15711-v30.xml emd-15711.xml emd-15711.xml | 17.4 KB 17.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15711_fsc.xml emd_15711_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_15711.png emd_15711.png | 120.8 KB | ||
| Others |  emd_15711_half_map_1.map.gz emd_15711_half_map_1.map.gz emd_15711_half_map_2.map.gz emd_15711_half_map_2.map.gz | 30.7 MB 30.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15711 http://ftp.pdbj.org/pub/emdb/structures/EMD-15711 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15711 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15711 | HTTPS FTP |
-Validation report
| Summary document |  emd_15711_validation.pdf.gz emd_15711_validation.pdf.gz | 859.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15711_full_validation.pdf.gz emd_15711_full_validation.pdf.gz | 858.7 KB | Display | |
| Data in XML |  emd_15711_validation.xml.gz emd_15711_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_15711_validation.cif.gz emd_15711_validation.cif.gz | 18.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15711 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15711 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15711 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15711 | HTTPS FTP |
-Related structure data
| Related structure data |  8ay5MC  8ay4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15711.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15711.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM map after pixel size scaling presented in 2-fold on Z (MRC standard) orientation. Micrographs were collected on lamellipodia of Cos7 cells infected with HRV2. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.64784 Å | ||||||||||||||||||||||||||||||||||||
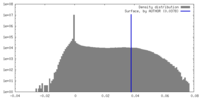
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: EM half map before pixel size scaling presented...
| File | emd_15711_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM half map before pixel size scaling presented in 2-fold on Z (MRC standard) orientation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: EM half map before pixel size scaling presented...
| File | emd_15711_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM half map before pixel size scaling presented in 2-fold on Z (MRC standard) orientation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : rhinovirus A2
| Entire | Name:  rhinovirus A2 rhinovirus A2 |
|---|---|
| Components |
|
-Supramolecule #1: rhinovirus A2
| Supramolecule | Name: rhinovirus A2 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 12130 / Sci species name: rhinovirus A2 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  rhinovirus A2 rhinovirus A2 |
| Molecular weight | Theoretical: 28.851441 KDa |
| Sequence | String: ALDAAETGHT SSVQPEDVIE TRYVQTSQTR DEMSLESFLG RSGCIHESKL EVTLANYNKE NFTVWAINLQ EMAQIRRKFE LFTYTRFDS EITLVPCISA LSQDIGHITM QYMYVPPGAP VPNSRDDYAW QSGTNASVFW QHGQAYPRFS LPFLSVASAY Y MFYDGYDE ...String: ALDAAETGHT SSVQPEDVIE TRYVQTSQTR DEMSLESFLG RSGCIHESKL EVTLANYNKE NFTVWAINLQ EMAQIRRKFE LFTYTRFDS EITLVPCISA LSQDIGHITM QYMYVPPGAP VPNSRDDYAW QSGTNASVFW QHGQAYPRFS LPFLSVASAY Y MFYDGYDE QDQNYGTANT NNMGSLCSRI VTEKHIHKVH IMTRIYHKAK HVKAWCPRPP RALEYTRAHR TNFKIEDRSI QT AIVTRPI ITTA UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  rhinovirus A2 rhinovirus A2 |
| Molecular weight | Theoretical: 27.899426 KDa |
| Sequence | String: RIIQITRGDS TITSQDVANA IVAYGVWPHY LSSKDASAID KPSQPDTSSN RFYTLRSVTW SSSSKGWWWK LPDALKDMGI FGENMFYHY LGRSGYTIHV QCNASKFHQG TLIVALIPEH QIASALHGNV NVGYNYTHPG ETGREVKAET RLNPDLQPTE E YWLNFDGT ...String: RIIQITRGDS TITSQDVANA IVAYGVWPHY LSSKDASAID KPSQPDTSSN RFYTLRSVTW SSSSKGWWWK LPDALKDMGI FGENMFYHY LGRSGYTIHV QCNASKFHQG TLIVALIPEH QIASALHGNV NVGYNYTHPG ETGREVKAET RLNPDLQPTE E YWLNFDGT LLGNITIFPH QFINLRSNNS ATIIAPYVNA VPMDSMRSHN NWSLVIIPIC PLETSSAINT IPITISISPM CA EFSGARA KRQ UniProtKB: Genome polyprotein |
-Macromolecule #3: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  rhinovirus A2 rhinovirus A2 |
| Molecular weight | Theoretical: 26.107793 KDa |
| Sequence | String: GLPVFITPGS GQFLTTDDFQ SPCALPWYHP TKEISIPGEV KNLVEICQVD SLVPINNTDT YINSENMYSV VLQSSINAPD KIFSIRTDV ASQPLATTLI GEISSYFTHW TGSLRFSFMF CGTANTTVKL LLAYTPPGIA EPTTRKDAML GTHVIWDVGL Q STISMVVP ...String: GLPVFITPGS GQFLTTDDFQ SPCALPWYHP TKEISIPGEV KNLVEICQVD SLVPINNTDT YINSENMYSV VLQSSINAPD KIFSIRTDV ASQPLATTLI GEISSYFTHW TGSLRFSFMF CGTANTTVKL LLAYTPPGIA EPTTRKDAML GTHVIWDVGL Q STISMVVP WISASHYRNT SPGRSTSGYI TCWYQTRLVI PPQTPPTARL LCFVSGCKDF CLRMARDTNL HLQSGAIAQ UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average exposure time: 10.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8ay5: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)