[English] 日本語
 Yorodumi
Yorodumi- EMDB-15664: Cryo-EM structure of yeast mitochondrial RNA polymerase transcrip... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of yeast mitochondrial RNA polymerase transcription initiation complex with 5-mer RNA, pppGpGpApApA (IC5) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | gene transcription / polymerase / RDRP / MTF1 / RPO41 / POLRMT / mtRNAP / DNA / transcription initiation / RNA polymerase / mitochondria / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationMitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial transcription factor activity / mitochondrial promoter sequence-specific DNA binding / transcription initiation at mitochondrial promoter / mitochondrial transcription / : / DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation / mitochondrial nucleoid ...Mitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial transcription factor activity / mitochondrial promoter sequence-specific DNA binding / transcription initiation at mitochondrial promoter / mitochondrial transcription / : / DNA replication, synthesis of primer / positive regulation of DNA-templated transcription, elongation / mitochondrial nucleoid / Transferases; Transferring one-carbon groups; Methyltransferases / methyltransferase activity / mitochondrial intermembrane space / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / methylation / mitochondrial matrix / mitochondrion / DNA binding / RNA binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
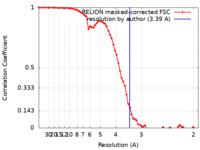
| Method | single particle reconstruction / cryo EM / Resolution: 3.39 Å | |||||||||
 Authors Authors | Goovaerts Q / Shen J / Patel SS / Das K | |||||||||
| Funding support |  Belgium, 1 items Belgium, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structures illustrate step-by-step mitochondrial transcription initiation. Authors: Quinten Goovaerts / Jiayu Shen / Brent De Wijngaert / Urmimala Basu / Smita S Patel / Kalyan Das /   Abstract: Transcription initiation is a key regulatory step in gene expression during which RNA polymerase (RNAP) initiates RNA synthesis de novo, and the synthesized RNA at a specific length triggers the ...Transcription initiation is a key regulatory step in gene expression during which RNA polymerase (RNAP) initiates RNA synthesis de novo, and the synthesized RNA at a specific length triggers the transition to the elongation phase. Mitochondria recruit a single-subunit RNAP and one or two auxiliary factors to initiate transcription. Previous studies have revealed the molecular architectures of yeast and human mitochondrial RNAP initiation complexes (ICs). Here we provide a comprehensive, stepwise mechanism of transcription initiation by solving high-resolution cryogenic electron microscopy (cryo-EM) structures of yeast mitochondrial RNAP and the transcription factor Mtf1 catalysing two- to eight-nucleotide RNA synthesis at single-nucleotide addition steps. The growing RNA-DNA is accommodated in the polymerase cleft by template scrunching and non-template reorganization, creating stressed intermediates. During early initiation, non-template strand scrunching and unscrunching destabilize the short two- and three-nucleotide RNAs, triggering abortive synthesis. Subsequently, the non-template reorganizes into a base-stacked staircase-like structure supporting processive five- to eight-nucleotide RNA synthesis. The expanded non-template staircase and highly scrunched template in IC8 destabilize the promoter interactions with Mtf1 to facilitate initiation bubble collapse and promoter escape for the transition from initiation to the elongation complex (EC). The series of transcription initiation steps, each guided by the interplay of multiple structural components, reveal a finely tuned mechanism for potential regulatory control. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15664.map.gz emd_15664.map.gz | 7.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15664-v30.xml emd-15664-v30.xml emd-15664.xml emd-15664.xml | 29.1 KB 29.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15664_fsc.xml emd_15664_fsc.xml | 6.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_15664.png emd_15664.png | 51.1 KB | ||
| Masks |  emd_15664_msk_1.map emd_15664_msk_1.map | 8.4 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15664.cif.gz emd-15664.cif.gz | 8.2 KB | ||
| Others |  emd_15664_half_map_1.map.gz emd_15664_half_map_1.map.gz emd_15664_half_map_2.map.gz emd_15664_half_map_2.map.gz | 7.7 MB 7.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15664 http://ftp.pdbj.org/pub/emdb/structures/EMD-15664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15664 | HTTPS FTP |
-Validation report
| Summary document |  emd_15664_validation.pdf.gz emd_15664_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15664_full_validation.pdf.gz emd_15664_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_15664_validation.xml.gz emd_15664_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_15664_validation.cif.gz emd_15664_validation.cif.gz | 15.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15664 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15664 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15664 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15664 | HTTPS FTP |
-Related structure data
| Related structure data |  8atvMC  8ap1C  8attC  8atwC  8c5sC  8c5uC  8q63C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15664.map.gz / Format: CCP4 / Size: 8.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15664.map.gz / Format: CCP4 / Size: 8.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.97 Å | ||||||||||||||||||||||||||||||||||||
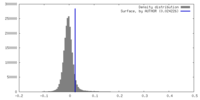
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15664_msk_1.map emd_15664_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
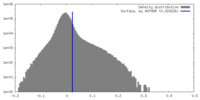
| Density Histograms |
-Half map: #2
| File | emd_15664_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
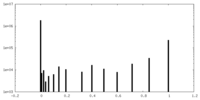
| Density Histograms |
-Half map: #1
| File | emd_15664_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
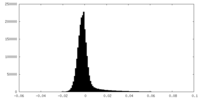
| Density Histograms |
- Sample components
Sample components
+Entire : yeast mitochondrial RNA polymerase transcription initiation compl...
+Supramolecule #1: yeast mitochondrial RNA polymerase transcription initiation compl...
+Supramolecule #2: DNA-directed RNA polymerase, mitochondrial
+Supramolecule #3: DNA (36-mer)
+Supramolecule #4: Mitochondrial transcription factor 1
+Supramolecule #5: RNA (pppGpGpApApA)
+Macromolecule #1: Mitochondrial transcription factor 1
+Macromolecule #2: DNA-directed RNA polymerase, mitochondrial
+Macromolecule #3: DNA (36-MER)
+Macromolecule #4: DNA (36-MER)
+Macromolecule #5: RNA (pppGpGpApApA)
+Macromolecule #6: GUANOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL |
|---|---|
| Buffer | pH: 7 Details: 50 mM Bis-Tris propane; 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 2 mM DTT |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.03 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 281.15 K / Instrument: LEICA EM GP / Details: 3 ul sample, back blotting for 12 seconds. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 602 / Average exposure time: 38.48 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 2.6 µm / Calibrated defocus min: 0.55 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 150000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Realspace refinement |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 140 / Target criteria: Correlation coefficient |
| Output model |  PDB-8atv: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)