+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Yeast RQC complex in state C | |||||||||
 Map data Map data | Yeast RQC complex in state C | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
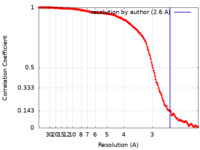
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Tesina P / Buschauer R / Beckmann R | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Molecular basis of eIF5A-dependent CAT tailing in eukaryotic ribosome-associated quality control. Authors: Petr Tesina / Shuhei Ebine / Robert Buschauer / Matthias Thoms / Yoshitaka Matsuo / Toshifumi Inada / Roland Beckmann /   Abstract: Ribosome-associated quality control (RQC) is a conserved process degrading potentially toxic truncated nascent peptides whose malfunction underlies neurodegeneration and proteostasis decline in aging. ...Ribosome-associated quality control (RQC) is a conserved process degrading potentially toxic truncated nascent peptides whose malfunction underlies neurodegeneration and proteostasis decline in aging. During RQC, dissociation of stalled ribosomes is followed by elongation of the nascent peptide with alanine and threonine residues, driven by Rqc2 independently of mRNA, the small ribosomal subunit and guanosine triphosphate (GTP)-hydrolyzing factors. The resulting CAT tails (carboxy-terminal tails) and ubiquitination by Ltn1 mark nascent peptides for proteasomal degradation. Here we present ten cryogenic electron microscopy (cryo-EM) structures, revealing the mechanistic basis of individual steps of the CAT tailing cycle covering initiation, decoding, peptidyl transfer, and tRNA translocation. We discovered eIF5A as a crucial eukaryotic RQC factor enabling peptidyl transfer. Moreover, we observed dynamic behavior of RQC factors and tRNAs allowing for processivity of the CAT tailing cycle without additional energy input. Together, these results elucidate key differences as well as common principles between CAT tailing and canonical translation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15432.map.gz emd_15432.map.gz | 20.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15432-v30.xml emd-15432-v30.xml emd-15432.xml emd-15432.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15432_fsc.xml emd_15432_fsc.xml | 15.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_15432.png emd_15432.png | 55.3 KB | ||
| Others |  emd_15432_half_map_1.map.gz emd_15432_half_map_1.map.gz emd_15432_half_map_2.map.gz emd_15432_half_map_2.map.gz | 322.7 MB 322.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15432 http://ftp.pdbj.org/pub/emdb/structures/EMD-15432 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15432 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15432 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15432.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15432.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast RQC complex in state C | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.059 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Yeast RQC complex in state C, half map A
| File | emd_15432_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast RQC complex in state C, half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Yeast RQC complex in state C, half map B
| File | emd_15432_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast RQC complex in state C, half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Yeast RQC complex in state C
| Entire | Name: Yeast RQC complex in state C |
|---|---|
| Components |
|
-Supramolecule #1: Yeast RQC complex in state C
| Supramolecule | Name: Yeast RQC complex in state C / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#54 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.4 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)