+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mot1:TBP:DNA - post hydrolysis state | |||||||||
 Map data Map data | main density | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Swi2/Snf2 protein / remodeler / transcription initiation / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationTBP-class protein binding / transcription initiation at RNA polymerase II promoter / helicase activity / transcription regulator complex / ATP hydrolysis activity / DNA binding / ATP binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Chaetomium thermophilum (fungus) / DNA molecule (others) Chaetomium thermophilum (fungus) / DNA molecule (others) | |||||||||
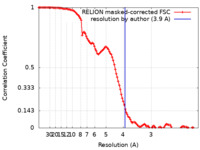
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Woike S / Eustermann S / Jung J / Wenzl SJ / Hagemann G / Bartho JD / Lammens K / Butryn A / Herzog F / Hopfner K-P | |||||||||
| Funding support |  Germany, European Union, 2 items Germany, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Structural basis for TBP displacement from TATA box DNA by the Swi2/Snf2 ATPase Mot1. Authors: Stephan Woike / Sebastian Eustermann / James Jung / Simon Josef Wenzl / Götz Hagemann / Joseph Bartho / Katja Lammens / Agata Butryn / Franz Herzog / Karl-Peter Hopfner /     Abstract: The Swi2/Snf2 family transcription regulator Modifier of Transcription 1 (Mot1) uses adenosine triphosphate (ATP) to dissociate and reallocate the TATA box-binding protein (TBP) from and between ...The Swi2/Snf2 family transcription regulator Modifier of Transcription 1 (Mot1) uses adenosine triphosphate (ATP) to dissociate and reallocate the TATA box-binding protein (TBP) from and between promoters. To reveal how Mot1 removes TBP from TATA box DNA, we determined cryogenic electron microscopy structures that capture different states of the remodeling reaction. The resulting molecular video reveals how Mot1 dissociates TBP in a process that, intriguingly, does not require DNA groove tracking. Instead, the motor grips DNA in the presence of ATP and swings back after ATP hydrolysis, moving TBP to a thermodynamically less stable position on DNA. Dislodged TBP is trapped by a chaperone element that blocks TBP's DNA binding site. Our results show how Swi2/Snf2 proteins can remodel protein-DNA complexes through DNA bending without processive DNA tracking and reveal mechanistic similarities to RNA gripping DEAD box helicases and RIG-I-like immune sensors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14562.map.gz emd_14562.map.gz | 117.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14562-v30.xml emd-14562-v30.xml emd-14562.xml emd-14562.xml | 21.5 KB 21.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14562_fsc.xml emd_14562_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_14562.png emd_14562.png | 52.4 KB | ||
| Masks |  emd_14562_msk_1.map emd_14562_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-14562.cif.gz emd-14562.cif.gz | 7.6 KB | ||
| Others |  emd_14562_half_map_1.map.gz emd_14562_half_map_1.map.gz emd_14562_half_map_2.map.gz emd_14562_half_map_2.map.gz | 98.4 MB 98.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14562 http://ftp.pdbj.org/pub/emdb/structures/EMD-14562 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14562 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14562 | HTTPS FTP |
-Related structure data
| Related structure data |  7z8sMC  7z7nC  7zb5C  7zkeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14562.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14562.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main density | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.059 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_14562_msk_1.map emd_14562_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map B
| File | emd_14562_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A
| File | emd_14562_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Mot1:TBP:DNA post hydrolysis complex
| Entire | Name: Mot1:TBP:DNA post hydrolysis complex |
|---|---|
| Components |
|
-Supramolecule #1: Mot1:TBP:DNA post hydrolysis complex
| Supramolecule | Name: Mot1:TBP:DNA post hydrolysis complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) Chaetomium thermophilum (fungus) |
-Macromolecule #1: DNA (36-MER)
| Macromolecule | Name: DNA (36-MER) / type: dna / ID: 1 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: DNA molecule (others) |
| Molecular weight | Theoretical: 11.155128 KDa |
| Sequence | String: (DC)(DG)(DG)(DC)(DC)(DG)(DG)(DG)(DC)(DG) (DC)(DC)(DC)(DG)(DG)(DC)(DA)(DT)(DG)(DG) (DC)(DG)(DG)(DC)(DC)(DT)(DA)(DT)(DA) (DA)(DA)(DA)(DG)(DG)(DG)(DC) |
-Macromolecule #2: DNA (36-MER)
| Macromolecule | Name: DNA (36-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: DNA molecule (others) |
| Molecular weight | Theoretical: 11.008016 KDa |
| Sequence | String: (DG)(DC)(DC)(DC)(DT)(DT)(DT)(DT)(DA)(DT) (DA)(DG)(DG)(DC)(DC)(DG)(DC)(DC)(DA)(DT) (DG)(DC)(DC)(DG)(DG)(DG)(DC)(DG)(DC) (DC)(DC)(DG)(DG)(DC)(DC)(DG) |
-Macromolecule #3: Putative tata-box binding protein
| Macromolecule | Name: Putative tata-box binding protein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 Chaetomium thermophilum (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 |
| Molecular weight | Theoretical: 29.676973 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGENLYFQG HMEAIQTHPA NAAQAKAFTA PGSLSFPGGA SEIANSTAPT NGASNGGQQQ GVQATSGAGV TPATPAATP GAGPAGPSGI TPTLQNIVAT VNLDCRLDLK TIALHARNAE YNPKRFAAVI MRIREPKTTA LIFASGKMVV T GAKSEDDS ...String: MGSSHHHHHH SSGENLYFQG HMEAIQTHPA NAAQAKAFTA PGSLSFPGGA SEIANSTAPT NGASNGGQQQ GVQATSGAGV TPATPAATP GAGPAGPSGI TPTLQNIVAT VNLDCRLDLK TIALHARNAE YNPKRFAAVI MRIREPKTTA LIFASGKMVV T GAKSEDDS KLASRKYARI IQKLGFNAKF TDFKIQNIVG SCDIKFPIRL EGLASKHHNF SSYEPELFPG LIYRMIKPKI VL LIFVSGK IVLTGAKVRE EIYQAFEMIY PVLQDFRKV UniProtKB: Putative tata-box binding protein |
-Macromolecule #4: Helicase-like protein
| Macromolecule | Name: Helicase-like protein / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 Chaetomium thermophilum (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 |
| Molecular weight | Theoretical: 211.832688 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MATRLDRLVT ILETGSTRLI RDTAVNQLAD WQKQHPEELF NLLSRVVPYL RHKDWETRTT AAKAIGKIIE NAPLYDPNAG QDEAAPEPT NGSFEVKKEE EKDVLEQDNF FRLESLDVAT IVKYGRPLLR GGPVDYNLAA LDPQKRLAHL KKTLNGRLGL L GRVFEDEE ...String: MATRLDRLVT ILETGSTRLI RDTAVNQLAD WQKQHPEELF NLLSRVVPYL RHKDWETRTT AAKAIGKIIE NAPLYDPNAG QDEAAPEPT NGSFEVKKEE EKDVLEQDNF FRLESLDVAT IVKYGRPLLR GGPVDYNLAA LDPQKRLAHL KKTLNGRLGL L GRVFEDEE MPVEQIASPI TPNDAAGANG VGRQDGASND NQSQAIDESK MSARQLNVLK RKRKREAQKA AQGKSGFGDL SL RRSTTAG SDAFGEDTPM PDADSKKNKL AEYFSLDRPE NTEEDTKIVS EFKGPVLPIK SEIEADDSLE GAEWPFERLC EFL KVDLFD PQWETRHGAA MGLREVIRVH GAGAGRRRGK TRKENNDLNR QWLDDLAYRL LCVLMLDKFT DYSSDTSVAP IRET VGQTL GAVLRHISVE SVHAIYRLLY CMVTQEDLPS EQNMWAVCHG GMVGLRYVVA VRKDLLLQDG DMIDGVVRCV MQGLG DIDD DVRSVSAATL IPMAKEFVMM RRSALDSLIN IVWESLSNLG DDLSASTGKI MDLLATLCSF PEVLEAMKVS ASQDEE RSF TLLVPRLYPF LRHTITSVRL AVLKALMTFA NLGGETSQGW LNGRILRLIF QNIIVERDQD TLNMSLELWT TLVRRLA AR DPAILADEFE AHAEPMMQLA LHPIGVPRHP IPMNPALFQK PSGGTYSLPG ASQTNSRRSS PPEGERATKR RRKSTKAE D VAPSTHTHDV DGHMIQGEVD LVGVDVLIRS RISAAKAMGL IMSFIPTPRL ASYDTAVLQA LSSPYASTQL AAAMVIDEY AKNCSTPEVA SRFIEPLQKI IDLERPSHYR DLVTYVQRVR SASQQLINLF RDHGKVSQGK LPTLAVVVQG EPEAGPGAFS IANAEKVVN EDFERLKRLM APGQRLIALP QLNEAREQTV EVIEEAKAAK EARDARIKAA AACALVAMKV LPKKPSPLIK A IMDSIKTE ENQELQSRSA ATIARLVQLF TESGRRGPAE KVVANLVKFS CVEVAETPEF PIHAHKTNVI LSMQKEEDRV DH PDAVKYA REAKAARITR RGAKEALEIL SKNFGAELLE RVPTLRTFME EPLVRAFSGD LPPEARDPEN AFGQEIVDAM SVI RTMTPT LHPALHPFVM QQVPLVIKAL RSDLSVFRYM AAKCMATICS VITVDGMTAL VEKVLPSINN PLDLSFRQGA IEVI YHLIA VMGDAILPYV IFLIVPVLGR MSDSDNQIRL IATTSFATLV KLVPLEAGIP DPPGLSEELL KGRDRERTFI AQLLD PKKI EPFKIPVAIK AELRSYQQEG VNWLAFLNKY HLHGILCDDM GLGKTLQTIC IVASDHHQRA EEFARTGAPE VRKLPS LII CPPTLSGHWQ QEIKTYAPFL TVTAYVGSPA ERRAMKDSLD KTDIVITSYD VCRNDIDVIE KYNWNYCVLD EGHLIKN PK AKITLAVKRL TSNHRLILTG TPIQNNVLEL WSLFDFLMPG FLGAEKVFLD RFAKPIANSR YSKASSKEQE AGALAIEA L HKQVLPFLLR RLKEEVLNDL PPKILQNYYC DLSDLQRKLF EDFTKREGKK ITETAGRDDK EAKQHIFQAL QYMRKLCNS PALVMKPGHK AYEDTQKYLA KHGTTLEDPI HAPKLGALRD LLVDCGIGVE GQESSDPLYT PIKPHRALIF CQMKEMLDMV QNTVLKQML PSVSYLRLDG SVEANKRQDI VNKFNSDPSY DVLLLTTSVG GLGLNLTGAD TVIFVEHDWN PQKDLQAMDR A HRIGQKKV VNVYRIITRG TLEEKILSLQ RFKIDVASTV VNQQNAGLAT MDTDQILDLF NLGESGPSLI TDNKESIEGR EE DMVDIET GDVRRPGKKA AWLEGLGELW DNAQYEESFD LDGFLKTMQA AAWSHPQFEK UniProtKB: TATA-binding protein-associated factor mot1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: Incubation with 1 mM ATP-gamma-S before size exclusion chromatography. 0.05% beta-octyl glucoside added before blotting. | ||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 7 sec. / Pretreatment - Atmosphere: AIR | ||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283 K / Instrument: LEICA EM GP Details: Incubation on the grid for 20 seconds before plunging.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)