[English] 日本語
 Yorodumi
Yorodumi- EMDB-14317: Cryo-EM reconstruction of the human 40S ribosomal subunit - Full map -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of the human 40S ribosomal subunit - Full map | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Ribosome / rRNA modifications / post-translational modifications / cryo-EM | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationoxidized pyrimidine DNA binding / response to TNF agonist / negative regulation of endoplasmic reticulum unfolded protein response / positive regulation of base-excision repair / positive regulation of respiratory burst involved in inflammatory response / positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage / positive regulation of gastrulation / protein tyrosine kinase inhibitor activity / positive regulation of endodeoxyribonuclease activity / nucleolus organization ...oxidized pyrimidine DNA binding / response to TNF agonist / negative regulation of endoplasmic reticulum unfolded protein response / positive regulation of base-excision repair / positive regulation of respiratory burst involved in inflammatory response / positive regulation of intrinsic apoptotic signaling pathway in response to DNA damage / positive regulation of gastrulation / protein tyrosine kinase inhibitor activity / positive regulation of endodeoxyribonuclease activity / nucleolus organization / IRE1-RACK1-PP2A complex / positive regulation of Golgi to plasma membrane protein transport / TNFR1-mediated ceramide production / negative regulation of DNA repair / negative regulation of RNA splicing / supercoiled DNA binding / NF-kappaB complex / cysteine-type endopeptidase activator activity involved in apoptotic process / neural crest cell differentiation / oxidized purine DNA binding / positive regulation of ubiquitin-protein transferase activity / negative regulation of intrinsic apoptotic signaling pathway in response to hydrogen peroxide / negative regulation of bicellular tight junction assembly / regulation of establishment of cell polarity / ubiquitin-like protein conjugating enzyme binding / rRNA modification in the nucleus and cytosol / negative regulation of phagocytosis / erythrocyte homeostasis / Formation of the ternary complex, and subsequently, the 43S complex / cytoplasmic side of rough endoplasmic reticulum membrane / negative regulation of ubiquitin protein ligase activity / protein kinase A binding / laminin receptor activity / ion channel inhibitor activity / Ribosomal scanning and start codon recognition / pigmentation / Translation initiation complex formation / positive regulation of mitochondrial depolarization / positive regulation of T cell receptor signaling pathway / fibroblast growth factor binding / negative regulation of Wnt signaling pathway / monocyte chemotaxis / TOR signaling / negative regulation of translational frameshifting / BH3 domain binding / positive regulation of activated T cell proliferation / Protein hydroxylation / SARS-CoV-1 modulates host translation machinery / regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / iron-sulfur cluster binding / cellular response to ethanol / regulation of cell division / mTORC1-mediated signalling / Peptide chain elongation / Selenocysteine synthesis / positive regulation of GTPase activity / Formation of a pool of free 40S subunits / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / Eukaryotic Translation Termination / negative regulation of ubiquitin-dependent protein catabolic process / protein serine/threonine kinase inhibitor activity / SRP-dependent cotranslational protein targeting to membrane / Response of EIF2AK4 (GCN2) to amino acid deficiency / ubiquitin ligase inhibitor activity / Viral mRNA Translation / negative regulation of respiratory burst involved in inflammatory response / positive regulation of signal transduction by p53 class mediator / negative regulation of protein binding / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression / Major pathway of rRNA processing in the nucleolus and cytosol / regulation of translational fidelity / phagocytic cup / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / positive regulation of intrinsic apoptotic signaling pathway / Protein methylation / spindle assembly / Nuclear events stimulated by ALK signaling in cancer / positive regulation of microtubule polymerization / positive regulation of cell cycle / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / rough endoplasmic reticulum / laminin binding / translation regulator activity / ribosomal small subunit export from nucleus / translation initiation factor binding / gastrulation / Maturation of protein E / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / Maturation of protein E / DNA-(apurinic or apyrimidinic site) endonuclease activity / ER Quality Control Compartment (ERQC) / signaling adaptor activity / MDM2/MDM4 family protein binding / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
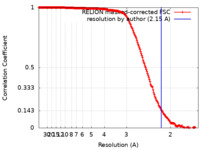
| Method | single particle reconstruction / cryo EM / Resolution: 2.15 Å | |||||||||||||||
 Authors Authors | Pellegrino S / Dent KC / Spikes T / Warren AJ | |||||||||||||||
| Funding support |  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Cryo-EM reconstruction of the human 40S ribosomal subunit at 2.15 Å resolution. Authors: Simone Pellegrino / Kyle C Dent / Tobias Spikes / Alan J Warren /  Abstract: The chemical modification of ribosomal RNA and proteins is critical for ribosome assembly, for protein synthesis and may drive ribosome specialisation in development and disease. However, the ...The chemical modification of ribosomal RNA and proteins is critical for ribosome assembly, for protein synthesis and may drive ribosome specialisation in development and disease. However, the inability to accurately visualise these modifications has limited mechanistic understanding of the role of these modifications in ribosome function. Here we report the 2.15 Å resolution cryo-EM reconstruction of the human 40S ribosomal subunit. We directly visualise post-transcriptional modifications within the 18S rRNA and four post-translational modifications of ribosomal proteins. Additionally, we interpret the solvation shells in the core regions of the 40S ribosomal subunit and reveal how potassium and magnesium ions establish both universally conserved and eukaryote-specific coordination to promote the stabilisation and folding of key ribosomal elements. This work provides unprecedented structural details for the human 40S ribosomal subunit that will serve as an important reference for unravelling the functional role of ribosomal RNA modifications. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14317.map.gz emd_14317.map.gz | 44.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14317-v30.xml emd-14317-v30.xml emd-14317.xml emd-14317.xml | 48.3 KB 48.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14317_fsc.xml emd_14317_fsc.xml | 18 KB | Display |  FSC data file FSC data file |
| Images |  emd_14317.png emd_14317.png | 59.5 KB | ||
| Filedesc metadata |  emd-14317.cif.gz emd-14317.cif.gz | 11.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14317 http://ftp.pdbj.org/pub/emdb/structures/EMD-14317 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14317 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14317 | HTTPS FTP |
-Related structure data
| Related structure data |  7r4xMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14317.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14317.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8267 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Human 40S ribosomal subunit
+Supramolecule #1: Human 40S ribosomal subunit
+Macromolecule #1: 60S ribosomal protein L41
+Macromolecule #3: 40S ribosomal protein S3a
+Macromolecule #4: 40S ribosomal protein S3
+Macromolecule #5: 40S ribosomal protein S4, X isoform
+Macromolecule #6: 40S ribosomal protein S5
+Macromolecule #7: 40S ribosomal protein S7
+Macromolecule #8: 40S ribosomal protein S8
+Macromolecule #9: 40S ribosomal protein S10
+Macromolecule #10: 40S ribosomal protein S11
+Macromolecule #11: 40S ribosomal protein S15
+Macromolecule #12: 40S ribosomal protein S16
+Macromolecule #13: 40S ribosomal protein S17
+Macromolecule #14: 40S ribosomal protein S18
+Macromolecule #15: 40S ribosomal protein S19
+Macromolecule #16: 40S ribosomal protein S20
+Macromolecule #17: 40S ribosomal protein S21
+Macromolecule #18: 40S ribosomal protein S23
+Macromolecule #19: 40S ribosomal protein S26
+Macromolecule #20: 40S ribosomal protein S28
+Macromolecule #21: 40S ribosomal protein S29
+Macromolecule #22: 40S ribosomal protein S2
+Macromolecule #23: 40S ribosomal protein S6
+Macromolecule #24: 40S ribosomal protein S9
+Macromolecule #25: 40S ribosomal protein S12
+Macromolecule #26: 40S ribosomal protein S13
+Macromolecule #27: 40S ribosomal protein S14
+Macromolecule #28: 40S ribosomal protein S15a
+Macromolecule #29: 40S ribosomal protein S24
+Macromolecule #30: 40S ribosomal protein S25
+Macromolecule #31: 40S ribosomal protein S27
+Macromolecule #32: 40S ribosomal protein S30
+Macromolecule #33: Ubiquitin-40S ribosomal protein S27a
+Macromolecule #34: Receptor of activated protein C kinase 1
+Macromolecule #35: 40S ribosomal protein SA
+Macromolecule #2: 18S ribosomal RNA
+Macromolecule #36: POTASSIUM ION
+Macromolecule #37: MAGNESIUM ION
+Macromolecule #38: ZINC ION
+Macromolecule #39: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.56 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)