+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of MuvB complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Quiescence / transcription / cell cycle | |||||||||
| Function / homology |  Function and homology information Function and homology informationMyb complex / CAF-1 complex / G0 to G1 transition / NURF complex / NuRD complex / regulation of cell fate specification / negative regulation of stem cell population maintenance / DNA replication-dependent chromatin assembly / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / ESC/E(Z) complex ...Myb complex / CAF-1 complex / G0 to G1 transition / NURF complex / NuRD complex / regulation of cell fate specification / negative regulation of stem cell population maintenance / DNA replication-dependent chromatin assembly / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / ESC/E(Z) complex / regulation of stem cell differentiation / Polo-like kinase mediated events / Transcription of E2F targets under negative control by DREAM complex / DNA biosynthetic process / ATPase complex / G1/S-Specific Transcription / histone deacetylase complex / Transcriptional Regulation by E2F6 / Sin3-type complex / positive regulation of stem cell population maintenance / RNA Polymerase I Transcription Initiation / G0 and Early G1 / Cyclin E associated events during G1/S transition / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / Cyclin A:Cdk2-associated events at S phase entry / Regulation of TP53 Activity through Acetylation / transcription repressor complex / Deposition of new CENPA-containing nucleosomes at the centromere / negative regulation of cell migration / Regulation of PTEN gene transcription / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / negative regulation of transforming growth factor beta receptor signaling pathway / brain development / PKMTs methylate histone lysines / Activation of anterior HOX genes in hindbrain development during early embryogenesis / histone deacetylase binding / HCMV Early Events / nucleosome assembly / gene expression / histone binding / Oxidative Stress Induced Senescence / Potential therapeutics for SARS / DNA replication / chromosome, telomeric region / regulation of cell cycle / RNA polymerase II cis-regulatory region sequence-specific DNA binding / chromatin remodeling / negative regulation of cell population proliferation / DNA repair / negative regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / DNA-templated transcription / positive regulation of DNA-templated transcription / chromatin / protein-containing complex binding / negative regulation of transcription by RNA polymerase II / protein-containing complex / DNA binding / nucleoplasm / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Koliopoulos MG / Alfieri C | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structure of a nucleosome-bound MuvB transcription factor complex reveals DNA remodelling. Authors: Marios G Koliopoulos / Reyhan Muhammad / Theodoros I Roumeliotis / Fabienne Beuron / Jyoti S Choudhary / Claudio Alfieri /  Abstract: Genes encoding the core cell cycle machinery are transcriptionally regulated by the MuvB family of protein complexes in a cell cycle-specific manner. Complexes of MuvB with the transcription factors ...Genes encoding the core cell cycle machinery are transcriptionally regulated by the MuvB family of protein complexes in a cell cycle-specific manner. Complexes of MuvB with the transcription factors B-MYB and FOXM1 activate mitotic genes during cell proliferation. The mechanisms of transcriptional regulation by these complexes are still poorly characterised. Here, we combine biochemical analysis and in vitro reconstitution, with structural analysis by cryo-electron microscopy and cross-linking mass spectrometry, to functionally examine these complexes. We find that the MuvB:B-MYB complex binds and remodels nucleosomes, thereby exposing nucleosomal DNA. This remodelling activity is supported by B-MYB which directly binds the remodelled DNA. Given the remodelling activity on the nucleosome, we propose that the MuvB:B-MYB complex functions as a pioneer transcription factor complex. In this work, we rationalise prior biochemical and cellular studies and provide a molecular framework of interactions on a protein complex that is key for cell cycle regulation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14239.map.gz emd_14239.map.gz | 2.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14239-v30.xml emd-14239-v30.xml emd-14239.xml emd-14239.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14239.png emd_14239.png | 99 KB | ||
| Filedesc metadata |  emd-14239.cif.gz emd-14239.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14239 http://ftp.pdbj.org/pub/emdb/structures/EMD-14239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14239 | HTTPS FTP |
-Related structure data
| Related structure data |  7r1dMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14239.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14239.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.134 Å | ||||||||||||||||||||||||||||||||||||
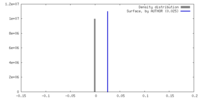
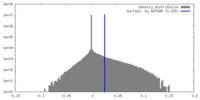
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : MuvB complex
| Entire | Name: MuvB complex |
|---|---|
| Components |
|
-Supramolecule #1: MuvB complex
| Supramolecule | Name: MuvB complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 180 KDa |
-Macromolecule #1: Protein lin-9 homolog
| Macromolecule | Name: Protein lin-9 homolog / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 62.03566 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MAELDQLPDE SSSAKALVSL KEGSLSNTWN EKYSSLQKTP VWKGRNTSSA VEMPFRNSKR SRLFSDEDDR QINTRSPKRN QRVAMVPQK FTATMSTPDK KASQKIGFRL RNLLKLPKAH KWCIYEWFYS NIDKPLFEGD NDFCVCLKES FPNLKTRKLT R VEWGKIRR ...String: MAELDQLPDE SSSAKALVSL KEGSLSNTWN EKYSSLQKTP VWKGRNTSSA VEMPFRNSKR SRLFSDEDDR QINTRSPKRN QRVAMVPQK FTATMSTPDK KASQKIGFRL RNLLKLPKAH KWCIYEWFYS NIDKPLFEGD NDFCVCLKES FPNLKTRKLT R VEWGKIRR LMGKPRRCSS AFFEEERSAL KQKRQKIRLL QQRKVADVSQ FKDLPDEIPL PLVIGTKVTA RLRGVHDGLF TG QIDAVDT LNATYRVTFD RTGLGTHTIP DYEVLSNEPH ETMPIAAFGQ KQRPSRFFMT PPRLHYTPPL QSPIIDNDPL LGQ SPWRSK ISGSDTETLG GFPVEFLIQV TRLSKILMIK KEHIKKLREM NTEAEKLKSY SMPISIEFQR RYATIVLELE QLNK DLNKV LHKVQQYCYE LAPDQGLQPA DQPTDMRRRC EEEAQEIVRH ANSSTGQPCV ENENLTDLIS RLTAILLQIK CLAEG GDLN SFEFKSLTDS LNDIKSTIDA SNISCFQNNV EIHVAHIQSG LSQMGNLHAF AANNTNRD UniProtKB: Protein lin-9 homolog |
-Macromolecule #2: Histone-binding protein RBBP4
| Macromolecule | Name: Histone-binding protein RBBP4 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.709527 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MADKEAAFDD AVEERVINEE YKIWKKNTPF LYDLVMTHAL EWPSLTAQWL PDVTRPEGKD FSIHRLVLGT HTSDEQNHLV IASVQLPND DAQFDASHYD SEKGEFGGFG SVSGKIEIEI KINHEGEVNR ARYMPQNPCI IATKTPSSDV LVFDYTKHPS K PDPSGECN ...String: MADKEAAFDD AVEERVINEE YKIWKKNTPF LYDLVMTHAL EWPSLTAQWL PDVTRPEGKD FSIHRLVLGT HTSDEQNHLV IASVQLPND DAQFDASHYD SEKGEFGGFG SVSGKIEIEI KINHEGEVNR ARYMPQNPCI IATKTPSSDV LVFDYTKHPS K PDPSGECN PDLRLRGHQK EGYGLSWNPN LSGHLLSASD DHTICLWDIS AVPKEGKVVD AKTIFTGHTA VVEDVSWHLL HE SLFGSVA DDQKLMIWDT RSNNTSKPSH SVDAHTAEVN CLSFNPYSEF ILATGSADKT VALWDLRNLK LKLHSFESHK DEI FQVQWS PHNETILASS GTDRRLNVWD LSKIGEEQSP EDAEDGPPEL LFIHGGHTAK ISDFSWNPNE PWVICSVSED NIMQ VWQMA ENIYNDEDPE GSVDPEGQGS UniProtKB: Histone-binding protein RBBP4 |
-Macromolecule #3: Protein lin-37 homolog
| Macromolecule | Name: Protein lin-37 homolog / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 29.37524 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MFPVKVKVEK SELEMAKARN QLDAVLQCLL EKSHMDRERL DEEAGKTPSD THNKDCSIAA TGKRPSARFP HQRRKKRREM DDGLAEGGP QRSNTYVIKL FDRSVDLAQF SENTPLYPIC RAWMRNSPSV RERECSPSSP LPPLPEDEEG SEVTNSKSRD V YKLPPPTP ...String: MFPVKVKVEK SELEMAKARN QLDAVLQCLL EKSHMDRERL DEEAGKTPSD THNKDCSIAA TGKRPSARFP HQRRKKRREM DDGLAEGGP QRSNTYVIKL FDRSVDLAQF SENTPLYPIC RAWMRNSPSV RERECSPSSP LPPLPEDEEG SEVTNSKSRD V YKLPPPTP PGPPGDACRS RIPSPLQPEM QGTPDDEPSE PEPSPSTLIY RNMQRWKRIR QRWKEASHRN QLRYSESMKI LR EMYERQG SALEVLFQ UniProtKB: Protein lin-37 homolog |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Material: COPPER |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)