+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-14084 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 26S proteasome Rpt1-RK -Ubp6-UbVS complex in the si state | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of ERAD pathway / SAGA complex localization to transcription regulatory region / Metalloprotease DUBs / negative regulation of proteasomal protein catabolic process / mitochondria-associated ubiquitin-dependent protein catabolic process / proteasome regulatory particle assembly / proteasome storage granule assembly / regulation of proteasomal ubiquitin-dependent protein catabolic process / transcription export complex 2 / protein deneddylation ...negative regulation of ERAD pathway / SAGA complex localization to transcription regulatory region / Metalloprotease DUBs / negative regulation of proteasomal protein catabolic process / mitochondria-associated ubiquitin-dependent protein catabolic process / proteasome regulatory particle assembly / proteasome storage granule assembly / regulation of proteasomal ubiquitin-dependent protein catabolic process / transcription export complex 2 / protein deneddylation / peroxisome fission / maintenance of DNA trinucleotide repeats / filamentous growth / COP9 signalosome / proteasome regulatory particle / protein-containing complex localization / mitochondrial fission / proteasome-activating activity / proteasome regulatory particle, lid subcomplex / proteasome regulatory particle, base subcomplex / hypothalamus gonadotrophin-releasing hormone neuron development / metal-dependent deubiquitinase activity / female meiosis I / positive regulation of protein monoubiquitination / fat pad development / mitochondrion transport along microtubule / K48-linked polyubiquitin modification-dependent protein binding / proteasome core complex assembly / nuclear outer membrane-endoplasmic reticulum membrane network / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / TNFR2 non-canonical NF-kB pathway / nonfunctional rRNA decay / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / proteasomal ubiquitin-independent protein catabolic process / Regulation of PTEN stability and activity / CDK-mediated phosphorylation and removal of Cdc6 / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / peptide catabolic process / KEAP1-NFE2L2 pathway / Neddylation / female gonad development / seminiferous tubule development / proteasome binding / Orc1 removal from chromatin / MAPK6/MAPK4 signaling / regulation of protein catabolic process / male meiosis I / proteasome storage granule / Antigen processing: Ubiquitination & Proteasome degradation / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / polyubiquitin modification-dependent protein binding / positive regulation of RNA polymerase II transcription preinitiation complex assembly / protein deubiquitination / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / endopeptidase activator activity / Ub-specific processing proteases / mRNA export from nucleus / proteasome assembly / threonine-type endopeptidase activity / proteasome core complex, alpha-subunit complex / energy homeostasis / regulation of neuron apoptotic process / neuron projection morphogenesis / enzyme regulator activity / regulation of proteasomal protein catabolic process / ERAD pathway / Maturation of protein E / Maturation of protein E / ER Quality Control Compartment (ERQC) / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Prevention of phagosomal-lysosomal fusion / IRAK2 mediated activation of TAK1 complex / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Neutrophil degranulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / PTK6 Regulates RTKs and Their Effectors AKT1 and DOK1 / protein folding chaperone / Regulation of TBK1, IKKε-mediated activation of IRF3, IRF7 upon TLR3 ligation / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / NOTCH2 Activation and Transmission of Signal to the Nucleus / TICAM1,TRAF6-dependent induction of TAK1 complex / TICAM1-dependent activation of IRF3/IRF7 / APC/C:Cdc20 mediated degradation of Cyclin B / Downregulation of ERBB4 signaling / Regulation of FZD by ubiquitination / APC-Cdc20 mediated degradation of Nek2A / p75NTR recruits signalling complexes / InlA-mediated entry of Listeria monocytogenes into host cells / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / TRAF6-mediated induction of TAK1 complex within TLR4 complex Similarity search - Function | ||||||||||||||||||
| Biological species |    Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 6.0 Å | ||||||||||||||||||
 Authors Authors | Hung KYS / Klumpe S / Eisele MR / Elsasser S / Geng TT / Cheng TC / Joshi T / Rudack T / Sakata E / Finley D | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Allosteric control of Ubp6 and the proteasome via a bidirectional switch. Authors: Ka Ying Sharon Hung / Sven Klumpe / Markus R Eisele / Suzanne Elsasser / Geng Tian / Shuangwu Sun / Jamie A Moroco / Tat Cheung Cheng / Tapan Joshi / Timo Seibel / Duco Van Dalen / Xin-Hua ...Authors: Ka Ying Sharon Hung / Sven Klumpe / Markus R Eisele / Suzanne Elsasser / Geng Tian / Shuangwu Sun / Jamie A Moroco / Tat Cheung Cheng / Tapan Joshi / Timo Seibel / Duco Van Dalen / Xin-Hua Feng / Ying Lu / Huib Ovaa / John R Engen / Byung-Hoon Lee / Till Rudack / Eri Sakata / Daniel Finley /      Abstract: The proteasome recognizes ubiquitinated proteins and can also edit ubiquitin marks, allowing substrates to be rejected based on ubiquitin chain topology. In yeast, editing is mediated by ...The proteasome recognizes ubiquitinated proteins and can also edit ubiquitin marks, allowing substrates to be rejected based on ubiquitin chain topology. In yeast, editing is mediated by deubiquitinating enzyme Ubp6. The proteasome activates Ubp6, whereas Ubp6 inhibits the proteasome through deubiquitination and a noncatalytic effect. Here, we report cryo-EM structures of the proteasome bound to Ubp6, based on which we identify mutants in Ubp6 and proteasome subunit Rpt1 that abrogate Ubp6 activation. The Ubp6 mutations define a conserved region that we term the ILR element. The ILR is found within the BL1 loop, which obstructs the catalytic groove in free Ubp6. Rpt1-ILR interaction opens the groove by rearranging not only BL1 but also a previously undescribed network of three interconnected active-site-blocking loops. Ubp6 activation and noncatalytic proteasome inhibition are linked in that they are eliminated by the same mutations. Ubp6 and ubiquitin together drive proteasomes into a unique conformation associated with proteasome inhibition. Thus, a multicomponent allosteric switch exerts simultaneous control over both Ubp6 and the proteasome. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14084.map.gz emd_14084.map.gz | 180.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14084-v30.xml emd-14084-v30.xml emd-14084.xml emd-14084.xml | 57.9 KB 57.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14084.png emd_14084.png | 106.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14084 http://ftp.pdbj.org/pub/emdb/structures/EMD-14084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14084 | HTTPS FTP |
-Related structure data
| Related structure data |  7qo5MC  7qo3C  7qo6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14084.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14084.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.38 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
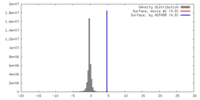
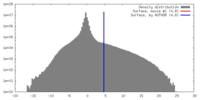
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : 26S proteasome Rpt1-RK -Ubp6-UbVS complex in the si state
+Supramolecule #1: 26S proteasome Rpt1-RK -Ubp6-UbVS complex in the si state
+Supramolecule #2: yeast Rpt1-RK 26S proteasome in si state
+Supramolecule #3: Ubp6-UbVS complex
+Macromolecule #1: Proteasome subunit alpha type-1
+Macromolecule #2: Proteasome subunit alpha type-2
+Macromolecule #3: Proteasome subunit alpha type-3
+Macromolecule #4: Proteasome subunit alpha type-4
+Macromolecule #5: Proteasome subunit alpha type-5
+Macromolecule #6: Proteasome subunit alpha type-6
+Macromolecule #7: Probable proteasome subunit alpha type-7
+Macromolecule #8: Proteasome subunit beta type-1
+Macromolecule #9: Proteasome subunit beta type-2
+Macromolecule #10: Proteasome subunit beta type-3
+Macromolecule #11: Proteasome subunit beta type-4
+Macromolecule #12: Proteasome subunit beta type-5
+Macromolecule #13: Proteasome subunit beta type-6
+Macromolecule #14: Proteasome subunit beta type-7
+Macromolecule #15: 26S proteasome regulatory subunit RPN10
+Macromolecule #16: Ubiquitin carboxyl-terminal hydrolase RPN11
+Macromolecule #17: 26S proteasome regulatory subunit RPN12
+Macromolecule #18: 26S proteasome regulatory subunit RPN13
+Macromolecule #19: 26S proteasome complex subunit SEM1
+Macromolecule #20: 26S proteasome regulatory subunit RPN1
+Macromolecule #21: 26S proteasome regulatory subunit RPN2
+Macromolecule #22: 26S proteasome regulatory subunit RPN3
+Macromolecule #23: 26S proteasome regulatory subunit RPN5
+Macromolecule #24: 26S proteasome regulatory subunit RPN6
+Macromolecule #25: 26S proteasome regulatory subunit RPN7
+Macromolecule #26: 26S proteasome regulatory subunit RPN8
+Macromolecule #27: 26S proteasome regulatory subunit RPN9
+Macromolecule #28: 26S proteasome regulatory subunit 7 homolog
+Macromolecule #29: 26S proteasome regulatory subunit 4 homolog
+Macromolecule #30: 26S proteasome regulatory subunit 6B homolog
+Macromolecule #31: 26S proteasome subunit RPT4
+Macromolecule #32: 26S proteasome regulatory subunit 6A
+Macromolecule #33: 26S proteasome regulatory subunit 8 homolog
+Macromolecule #34: Ubiquitin carboxyl-terminal hydrolase 6
+Macromolecule #35: Polyubiquitin-B
+Macromolecule #36: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #37: MAGNESIUM ION
+Macromolecule #38: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 6.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 64766 |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)