+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Infectious mouse-adapted RML scrapie prion fibril purified from terminally-infected mouse brains | |||||||||||||||
 マップデータ マップデータ | Ex vivo RML prion fibril | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | Prion / Amyloid / PrP / Prion protein / mouse RML scrapie strain / ex vivo prion / PROTEIN FIBRIL | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process / regulation of glutamate receptor signaling pathway / lamin binding / aspartic-type endopeptidase inhibitor activity / regulation of calcium ion import across plasma membrane / positive regulation of glutamate receptor signaling pathway / glycosaminoglycan binding / type 5 metabotropic glutamate receptor binding / ATP-dependent protein binding ...Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process / regulation of glutamate receptor signaling pathway / lamin binding / aspartic-type endopeptidase inhibitor activity / regulation of calcium ion import across plasma membrane / positive regulation of glutamate receptor signaling pathway / glycosaminoglycan binding / type 5 metabotropic glutamate receptor binding / ATP-dependent protein binding / negative regulation of interleukin-17 production / cupric ion binding / regulation of potassium ion transmembrane transport / negative regulation of dendritic spine maintenance / nucleobase-containing compound metabolic process / negative regulation of calcineurin-NFAT signaling cascade / negative regulation of interleukin-2 production / negative regulation of T cell receptor signaling pathway / negative regulation of activated T cell proliferation / negative regulation of amyloid-beta formation / cuprous ion binding / response to amyloid-beta / negative regulation of type II interferon production / negative regulation of long-term synaptic potentiation / intracellular copper ion homeostasis / positive regulation of protein targeting to membrane / response to cadmium ion / side of membrane / inclusion body / neuron projection maintenance / positive regulation of calcium-mediated signaling / cellular response to copper ion / molecular function activator activity / positive regulation of protein localization to plasma membrane / molecular condensate scaffold activity / protein destabilization / protein homooligomerization / cellular response to xenobiotic stimulus / cellular response to amyloid-beta / terminal bouton / positive regulation of neuron apoptotic process / signaling receptor activity / regulation of protein localization / protein-folding chaperone binding / amyloid-beta binding / response to oxidative stress / protease binding / nuclear membrane / microtubule binding / molecular adaptor activity / transmembrane transporter binding / learning or memory / postsynaptic density / intracellular signal transduction / membrane raft / copper ion binding / dendrite / negative regulation of apoptotic process / protein-containing complex binding / cell surface / endoplasmic reticulum / negative regulation of transcription by RNA polymerase II / Golgi apparatus / metal ion binding / identical protein binding / membrane / plasma membrane / cytosol 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |  | |||||||||||||||
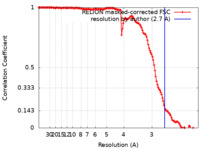
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 2.7 Å | |||||||||||||||
 データ登録者 データ登録者 | Manka SW / Zhang W | |||||||||||||||
| 資金援助 |  英国, 4件 英国, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2022 ジャーナル: Nat Commun / 年: 2022タイトル: 2.7 Å cryo-EM structure of ex vivo RML prion fibrils. 著者: Szymon W Manka / Wenjuan Zhang / Adam Wenborn / Jemma Betts / Susan Joiner / Helen R Saibil / John Collinge / Jonathan D F Wadsworth /  要旨: Mammalian prions propagate as distinct strains and are composed of multichain assemblies of misfolded host-encoded prion protein (PrP). Here, we present a near-atomic resolution cryo-EM structure of ...Mammalian prions propagate as distinct strains and are composed of multichain assemblies of misfolded host-encoded prion protein (PrP). Here, we present a near-atomic resolution cryo-EM structure of PrP fibrils present in highly infectious prion rod preparations isolated from the brains of RML prion-infected mice. We found that prion rods comprise single-protofilament helical amyloid fibrils that coexist with twisted pairs of the same protofilaments. Each rung of the protofilament is formed by a single PrP monomer with the ordered core comprising PrP residues 94-225, which folds to create two asymmetric lobes with the N-linked glycans and the glycosylphosphatidylinositol anchor projecting from the C-terminal lobe. The overall architecture is comparable to that of recently reported PrP fibrils isolated from the brain of hamsters infected with the 263K prion strain. However, there are marked conformational variations that could result from differences in PrP sequence and/or represent distinguishing features of the distinct prion strains. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_13989.map.gz emd_13989.map.gz | 10.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-13989-v30.xml emd-13989-v30.xml emd-13989.xml emd-13989.xml | 12.7 KB 12.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_13989_fsc.xml emd_13989_fsc.xml | 13.5 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_13989.png emd_13989.png | 89.7 KB | ||
| Filedesc metadata |  emd-13989.cif.gz emd-13989.cif.gz | 5.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13989 http://ftp.pdbj.org/pub/emdb/structures/EMD-13989 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13989 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13989 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_13989_validation.pdf.gz emd_13989_validation.pdf.gz | 351.3 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_13989_full_validation.pdf.gz emd_13989_full_validation.pdf.gz | 350.9 KB | 表示 | |
| XML形式データ |  emd_13989_validation.xml.gz emd_13989_validation.xml.gz | 13.6 KB | 表示 | |
| CIF形式データ |  emd_13989_validation.cif.gz emd_13989_validation.cif.gz | 18.6 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13989 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13989 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13989 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13989 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7qigMC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
| 電子顕微鏡画像生データ |  EMPIAR-10992 (タイトル: Infectious mouse-adapted RML scrapie prion fibrils purified from terminally-infected mouse brains EMPIAR-10992 (タイトル: Infectious mouse-adapted RML scrapie prion fibrils purified from terminally-infected mouse brainsData size: 1.0 TB Data #1: Aligned multi-frame micrographs of RML prion fibrils [micrographs - single frame]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_13989.map.gz / 形式: CCP4 / 大きさ: 216 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_13989.map.gz / 形式: CCP4 / 大きさ: 216 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Ex vivo RML prion fibril | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.067 Å | ||||||||||||||||||||||||||||||||||||
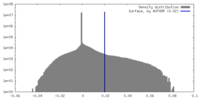
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Amyloid fibril of mouse PrP from RML--infected mouse brain
| 全体 | 名称: Amyloid fibril of mouse PrP from RML--infected mouse brain |
|---|---|
| 要素 |
|
-超分子 #1: Amyloid fibril of mouse PrP from RML--infected mouse brain
| 超分子 | 名称: Amyloid fibril of mouse PrP from RML--infected mouse brain タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Major prion protein
| 分子 | 名称: Major prion protein / タイプ: protein_or_peptide / ID: 1 / コピー数: 3 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 15.290103 KDa |
| 配列 | 文字列: THNQWNKPSK PKTNLKHVAG AAAAGAVVGG LGGYMLGSAM SRPMIHFGND WEDRYYRENM YRYPNQVYYR PVDQYSNQNN FVHDCVNIT IKQHTVTTTT KGENFTETDV KMMERVVEQM CVTQYQKESQ AYY UniProtKB: Major prion protein |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 緩衝液 | pH: 6.8 |
|---|---|
| グリッド | モデル: C-flat-2/2 / 材質: COPPER / メッシュ: 300 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 45 sec. |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 49.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.0 µm / 最小 デフォーカス(公称値): 1.5 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | 空間: REAL / プロトコル: AB INITIO MODEL |
|---|---|
| 得られたモデル |  PDB-7qig: |
 ムービー
ムービー コントローラー
コントローラー






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)