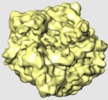
登録情報 データベース : EMDB / ID : EMD-1365タイトル Locking and unlocking of ribosomal motions. Cryo-EM map of E.coli 70S ribosome 試料 : EF-G bound Release Complex in the presence of Puromycin, GDP and fus複合体 : Release Complexリガンド : Puromycinリガンド : EF-Gリガンド : GDPリガンド : Fusidic acid機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Escherichia coli (大腸菌) / synthetic construct (人工物) 手法 / / 解像度 : 11.75 Å Mikel V / Andrey Z / Sengupta J / Rawat U / Ehrenberg M / Frank J ジャーナル : Cell / 年 : 2003タイトル : Locking and unlocking of ribosomal motions.著者 : Mikel Valle / Andrey Zavialov / Jayati Sengupta / Urmila Rawat / Måns Ehrenberg / Joachim Frank / 要旨 : During the ribosomal translocation, the binding of elongation factor G (EF-G) to the pretranslocational ribosome leads to a ratchet-like rotation of the 30S subunit relative to the 50S subunit in the ... During the ribosomal translocation, the binding of elongation factor G (EF-G) to the pretranslocational ribosome leads to a ratchet-like rotation of the 30S subunit relative to the 50S subunit in the direction of the mRNA movement. By means of cryo-electron microscopy we observe that this rotation is accompanied by a 20 A movement of the L1 stalk of the 50S subunit, implying that this region is involved in the translocation of deacylated tRNAs from the P to the E site. These ribosomal motions can occur only when the P-site tRNA is deacylated. Prior to peptidyl-transfer to the A-site tRNA or peptide removal, the presence of the charged P-site tRNA locks the ribosome and prohibits both of these motions. 履歴 登録 2007年5月21日 - ヘッダ(付随情報) 公開 2007年5月24日 - マップ公開 2007年5月24日 - 更新 2012年11月7日 - 現状 2012年11月7日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 機能・相同性情報
機能・相同性情報
 データ登録者
データ登録者 引用
引用 ジャーナル: Cell / 年: 2003
ジャーナル: Cell / 年: 2003
 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_1365.map.gz
emd_1365.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-1365-v30.xml
emd-1365-v30.xml emd-1365.xml
emd-1365.xml EMDBヘッダ
EMDBヘッダ 1365.gif
1365.gif http://ftp.pdbj.org/pub/emdb/structures/EMD-1365
http://ftp.pdbj.org/pub/emdb/structures/EMD-1365 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1365
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1365 emd_1365_validation.pdf.gz
emd_1365_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_1365_full_validation.pdf.gz
emd_1365_full_validation.pdf.gz emd_1365_validation.xml.gz
emd_1365_validation.xml.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1365
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1365 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1365
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1365 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_1365.map.gz / 形式: CCP4 / 大きさ: 8.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_1365.map.gz / 形式: CCP4 / 大きさ: 8.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素

 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN 画像解析
画像解析 ムービー
ムービー コントローラー
コントローラー






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















