[English] 日本語
 Yorodumi
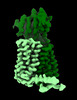
Yorodumi- EMDB-13544: HsPepT2 bound to Ala-Phe in the inward facing partially occluded ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13544 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HsPepT2 bound to Ala-Phe in the inward facing partially occluded conformation | |||||||||
 Map data Map data | post processed in Phenix. Used for refinement | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HsPepT2 / PepT2 / Peptide transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationtripeptide import across plasma membrane / dipeptide transport / peptidoglycan transport / dipeptide import across plasma membrane / tripeptide transmembrane transporter activity / peptide:proton symporter activity / antibacterial innate immune response / dipeptide transmembrane transporter activity / regulation of nucleotide-binding domain, leucine rich repeat containing receptor signaling pathway / xenobiotic detoxification by transmembrane export across the plasma membrane ...tripeptide import across plasma membrane / dipeptide transport / peptidoglycan transport / dipeptide import across plasma membrane / tripeptide transmembrane transporter activity / peptide:proton symporter activity / antibacterial innate immune response / dipeptide transmembrane transporter activity / regulation of nucleotide-binding domain, leucine rich repeat containing receptor signaling pathway / xenobiotic detoxification by transmembrane export across the plasma membrane / renal absorption / xenobiotic transport / transport across blood-brain barrier / phagocytic vesicle membrane / protein transport / apical plasma membrane / extracellular exosome / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
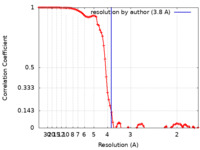
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Killer M / Wald J | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Structural snapshots of human PepT1 and PepT2 reveal mechanistic insights into substrate and drug transport across epithelial membranes. Authors: Maxime Killer / Jiri Wald / Joanna Pieprzyk / Thomas C Marlovits / Christian Löw /  Abstract: The uptake of peptides in mammals plays a crucial role in nutrition and inflammatory diseases. This process is mediated by promiscuous transporters of the solute carrier family 15, which form part of ...The uptake of peptides in mammals plays a crucial role in nutrition and inflammatory diseases. This process is mediated by promiscuous transporters of the solute carrier family 15, which form part of the major facilitator superfamily. Besides the uptake of short peptides, peptide transporter 1 (PepT1) is a highly abundant drug transporter in the intestine and represents a major route for oral drug delivery. PepT2 also allows renal drug reabsorption from ultrafiltration and brain-to-blood efflux of neurotoxic compounds. Here, we present cryogenic electron microscopy (cryo-EM) structures of human PepT1 and PepT2 captured in four different states throughout the transport cycle. The structures reveal the architecture of human peptide transporters and provide mechanistic insights into substrate recognition and conformational transitions during transport. This may support future drug design efforts to increase the bioavailability of different drugs in the human body. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13544.map.gz emd_13544.map.gz | 57.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13544-v30.xml emd-13544-v30.xml emd-13544.xml emd-13544.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13544_fsc.xml emd_13544_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_13544.png emd_13544.png | 71.3 KB | ||
| Masks |  emd_13544_msk_1.map emd_13544_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13544.cif.gz emd-13544.cif.gz | 5.7 KB | ||
| Others |  emd_13544_additional_1.map.gz emd_13544_additional_1.map.gz emd_13544_half_map_1.map.gz emd_13544_half_map_1.map.gz emd_13544_half_map_2.map.gz emd_13544_half_map_2.map.gz | 56.5 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13544 http://ftp.pdbj.org/pub/emdb/structures/EMD-13544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13544 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13544 | HTTPS FTP |
-Related structure data
| Related structure data |  7pmyMC  7pmwC  7pmxC  7pn1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13544.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13544.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | post processed in Phenix. Used for refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13544_msk_1.map emd_13544_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
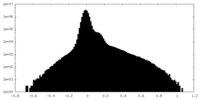
| Density Histograms |
-Additional map: #1
| File | emd_13544_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
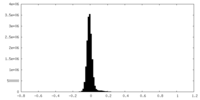
| Density Histograms |
-Half map: #2
| File | emd_13544_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
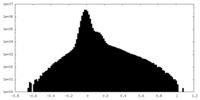
| Density Histograms |
-Half map: #1
| File | emd_13544_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human PepT2
| Entire | Name: Human PepT2 |
|---|---|
| Components |
|
-Supramolecule #1: Human PepT2
| Supramolecule | Name: Human PepT2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #2: ALA-PHE
| Supramolecule | Name: ALA-PHE / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 15 member 2
| Macromolecule | Name: Solute carrier family 15 member 2 / type: protein_or_peptide / ID: 1 / Details: HsPepT2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 81.861125 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MNPFQKNESK ETLFSPVSIE EVPPRPPSPP KKPSPTICGS NYPLSIAFIV VNEFCERFSY YGMKAVLILY FLYFLHWNED TSTSIYHAF SSLCYFTPIL GAAIADSWLG KFKTIIYLSL VYVLGHVIKS LGALPILGGQ VVHTVLSLIG LSLIALGTGG I KPCVAAFG ...String: MNPFQKNESK ETLFSPVSIE EVPPRPPSPP KKPSPTICGS NYPLSIAFIV VNEFCERFSY YGMKAVLILY FLYFLHWNED TSTSIYHAF SSLCYFTPIL GAAIADSWLG KFKTIIYLSL VYVLGHVIKS LGALPILGGQ VVHTVLSLIG LSLIALGTGG I KPCVAAFG GDQFEEKHAE ERTRYFSVFY LSINAGSLIS TFITPMLRGD VQCFGEDCYA LAFGVPGLLM VIALVVFAMG SK IYNKPPP EGNIVAQVFK CIWFAISNRF KNRSGDIPKR QHWLDWAAEK YPKQLIMDVK ALTRVLFLYI PLPMFWALLD QQG SRWTLQ AIRMNRNLGF FVLQPDQMQV LNPLLVLIFI PLFDFVIYRL VSKCGINFSS LRKMAVGMIL ACLAFAVAAA VEIK INEMA PAQPGPQEVF LQVLNLADDE VKVTVVGNEN NSLLIESIKS FQKTPHYSKL HLKTKSQDFH FHLKYHNLSL YTEHS VQEK NWYSLVIRED GNSISSMMVK DTESRTTNGM TTVRFVNTLH KDVNISLSTD TSLNVGEDYG VSAYRTVQRG EYPAVH CRT EDKNFSLNLG LLDFGAAYLF VITNNTNQGL QAWKIEDIPA NKMSIAWQLP QYALVTAGEV MFSVTGLEFS YSQAPSS MK SVLQAAWLLT IAVGNIIVLV VAQFSGLVQW AEFILFSCLL LVICLIFSIM GYYYVPVKTE DMRGPADKHI PHIQGNMI K LETKKTKL UniProtKB: Solute carrier family 15 member 2 |
-Macromolecule #2: ALA-PHE
| Macromolecule | Name: ALA-PHE / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 236.267 Da |
| Sequence | String: AF |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: PROPANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 81.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)