[English] 日本語
 Yorodumi
Yorodumi- EMDB-13296: RuvAB branch migration motor complexed to the Holliday junction -... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RuvAB branch migration motor complexed to the Holliday junction - RuvB AAA+ state s3 [t2 dataset] | |||||||||
 Map data Map data | RuvB AAA hexamer state s3 (t2) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA recombination / DNA repair / branch migration / Holliday junction / helicase / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationHolliday junction helicase complex / Holliday junction resolvase complex / four-way junction helicase activity / SOS response / four-way junction DNA binding / DNA recombination / DNA helicase / DNA repair / ATP hydrolysis activity / ATP binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Streptococcus thermophilus (bacteria) / Streptococcus thermophilus (bacteria) /  Salmonella typhimurium (bacteria) / synthetic construct (others) Salmonella typhimurium (bacteria) / synthetic construct (others) | |||||||||
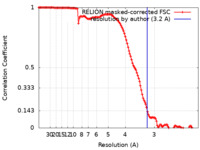
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Wald J / Marlovits TC | |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Mechanism of AAA+ ATPase-mediated RuvAB-Holliday junction branch migration. Authors: Jiri Wald / Dirk Fahrenkamp / Nikolaus Goessweiner-Mohr / Wolfgang Lugmayr / Luciano Ciccarelli / Oliver Vesper / Thomas C Marlovits /    Abstract: The Holliday junction is a key intermediate formed during DNA recombination across all kingdoms of life. In bacteria, the Holliday junction is processed by two homo-hexameric AAA+ ATPase RuvB motors, ...The Holliday junction is a key intermediate formed during DNA recombination across all kingdoms of life. In bacteria, the Holliday junction is processed by two homo-hexameric AAA+ ATPase RuvB motors, which assemble together with the RuvA-Holliday junction complex to energize the strand-exchange reaction. Despite its importance for chromosome maintenance, the structure and mechanism by which this complex facilitates branch migration are unknown. Here, using time-resolved cryo-electron microscopy, we obtained structures of the ATP-hydrolysing RuvAB complex in seven distinct conformational states, captured during assembly and processing of a Holliday junction. Five structures together resolve the complete nucleotide cycle and reveal the spatiotemporal relationship between ATP hydrolysis, nucleotide exchange and context-specific conformational changes in RuvB. Coordinated motions in a converter formed by DNA-disengaged RuvB subunits stimulate hydrolysis and nucleotide exchange. Immobilization of the converter enables RuvB to convert the ATP-contained energy into a lever motion, which generates the pulling force driving the branch migration. We show that RuvB motors rotate together with the DNA substrate, which, together with a progressing nucleotide cycle, forms the mechanistic basis for DNA recombination by continuous branch migration. Together, our data decipher the molecular principles of homologous recombination by the RuvAB complex, elucidate discrete and sequential transition-state intermediates for chemo-mechanical coupling of hexameric AAA+ motors and provide a blueprint for the design of state-specific compounds targeting AAA+ motors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13296.map.gz emd_13296.map.gz | 9.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13296-v30.xml emd-13296-v30.xml emd-13296.xml emd-13296.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13296_fsc.xml emd_13296_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_13296.png emd_13296.png | 91.9 KB | ||
| Filedesc metadata |  emd-13296.cif.gz emd-13296.cif.gz | 11.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13296 http://ftp.pdbj.org/pub/emdb/structures/EMD-13296 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13296 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13296 | HTTPS FTP |
-Related structure data
| Related structure data |  7pbnMC  7pblC  7pbmC  7pboC  7pbpC  7pbqC  7pbrC  7pbsC  7pbtC  7pbuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13296.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13296.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RuvB AAA hexamer state s3 (t2) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.09 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : RuvAB branch migration motor complexed to the Holliday junction -...
| Entire | Name: RuvAB branch migration motor complexed to the Holliday junction - RuvB motor state s3 [t2 dataset] |
|---|---|
| Components |
|
-Supramolecule #1: RuvAB branch migration motor complexed to the Holliday junction -...
| Supramolecule | Name: RuvAB branch migration motor complexed to the Holliday junction - RuvB motor state s3 [t2 dataset] type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 / Details: RuvB helicase |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
| Molecular weight | Theoretical: 220 KDa |
-Macromolecule #1: Holliday junction ATP-dependent DNA helicase RuvB
| Macromolecule | Name: Holliday junction ATP-dependent DNA helicase RuvB / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
| Molecular weight | Theoretical: 35.447508 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: TLRPQYFKEY IGQDKVKDQL KIFIEAAKLR DEALDHTLLF GPPGLGKTTM AFVIANEMGV NLKQTSGPAI EKAGDLVAIL NDLEPGDIL FIDEIHRMPM AVEEVLYSAM EDYYIDIMIG AGETSRSVHL DLPPFTLVGA TTRAGMLSNP LRARFGINGH M EYYELPDL ...String: TLRPQYFKEY IGQDKVKDQL KIFIEAAKLR DEALDHTLLF GPPGLGKTTM AFVIANEMGV NLKQTSGPAI EKAGDLVAIL NDLEPGDIL FIDEIHRMPM AVEEVLYSAM EDYYIDIMIG AGETSRSVHL DLPPFTLVGA TTRAGMLSNP LRARFGINGH M EYYELPDL TEIVERTSEI FEMTITPEAA LELARRSRGT PRIANRLLKR VRDYAQIMGD GVIDDKIADQ ALTMLDVDHE GL DYVDQKI LRTMIEMYGG GPVGLGTLSV NIAEERETVE DMYEPYLIQK GFIMRTRTGR VATAKAYEHM GYDYTRDN UniProtKB: Holliday junction branch migration complex subunit RuvB |
-Macromolecule #2: Holliday junction ATP-dependent DNA helicase RuvA
| Macromolecule | Name: Holliday junction ATP-dependent DNA helicase RuvA / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) |
| Molecular weight | Theoretical: 5.105734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SEDAEQEAVA ALVALGYKPQ EASRMVSKIA RPDASSETLI RDALRAAL UniProtKB: Holliday junction branch migration complex subunit RuvA |
-Macromolecule #3: Holliday junction ATP-dependent DNA helicase RuvA
| Macromolecule | Name: Holliday junction ATP-dependent DNA helicase RuvA / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) |
| Molecular weight | Theoretical: 5.442131 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DAEQEAVAAL VALGYKPQEA SRMVSKIARP DASSETLIRD ALRAALHHHH UniProtKB: Holliday junction branch migration complex subunit RuvA |
-Macromolecule #4: random DNA sequence
| Macromolecule | Name: random DNA sequence / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.643037 KDa |
| Sequence | String: (DG)(DA)(DA)(DC)(DC)(DT)(DT)(DC)(DG)(DA) (DG)(DG)(DA)(DA)(DG) |
-Macromolecule #5: random DNA sequence
| Macromolecule | Name: random DNA sequence / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.535946 KDa |
| Sequence | String: (DC)(DT)(DT)(DC)(DC)(DT)(DC)(DG)(DA)(DA) (DG)(DG)(DT)(DT)(DC) |
-Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 4 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #7: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 7 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #8: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 8 / Number of copies: 2 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 0.15 |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | in-vitro reconstituted freshly before vitrification |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Number real images: 30083 / Average exposure time: 5.0 sec. / Average electron dose: 30.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|---|
| Output model |  PDB-7pbn: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)