[English] 日本語
 Yorodumi
Yorodumi- EMDB-12963: Structure of the outer-membrane core complex (outer ring) from a ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12963 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the outer-membrane core complex (outer ring) from a conjugative type IV secretion system | |||||||||
 Map data Map data | The structure of the outer-membrane core complex (outer ring) from a conjugative type IV secretion system | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Type IV secretion system / F plasmid / outer-membrane core complex / conjugation / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationPilus assembly TraK / Type-F conjugative transfer system secretin TraK / : / TraK N-terminal domain / TraK C-terminal domain / Type IV conjugative transfer system protein TraV / Type IV conjugative transfer system lipoprotein (TraV) / Prokaryotic membrane lipoprotein lipid attachment site profile. Similarity search - Domain/homology | |||||||||
| Biological species |  Salmonella enterica (bacteria) / Salmonella enterica (bacteria) /  Salmonella enterica subsp. salamae serovar 58:l,z13,z28:z6 (bacteria) Salmonella enterica subsp. salamae serovar 58:l,z13,z28:z6 (bacteria) | |||||||||
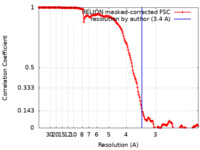
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Amin H / Ilangovan A / Costa TRD | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Architecture of the outer-membrane core complex from a conjugative type IV secretion system. Authors: Himani Amin / Aravindan Ilangovan / Tiago R D Costa /  Abstract: Conjugation is one of the most important processes that bacteria utilize to spread antibiotic resistance genes among bacterial populations. Interbacterial DNA transfer requires a large double ...Conjugation is one of the most important processes that bacteria utilize to spread antibiotic resistance genes among bacterial populations. Interbacterial DNA transfer requires a large double membrane-spanning nanomachine called the type 4 secretion system (T4SS) made up of the inner-membrane complex (IMC), the outer-membrane core complex (OMCC) and the conjugative pilus. The iconic F plasmid-encoded T4SS has been central in understanding conjugation for several decades, however atomic details of its structure are not known. Here, we report the structure of a complete conjugative OMCC encoded by the pED208 plasmid from E. coli, solved by cryo-electron microscopy at 3.3 Å resolution. This 2.1 MDa complex has a unique arrangement with two radial concentric rings, each having a different symmetry eventually contributing to remarkable differences in protein stoichiometry and flexibility in comparison to other OMCCs. Our structure suggests that F-OMCC is a highly dynamic complex, with implications for pilus extension and retraction during conjugation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12963.map.gz emd_12963.map.gz | 391.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12963-v30.xml emd-12963-v30.xml emd-12963.xml emd-12963.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12963_fsc.xml emd_12963_fsc.xml | 16.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_12963.png emd_12963.png | 63.9 KB | ||
| Filedesc metadata |  emd-12963.cif.gz emd-12963.cif.gz | 5.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12963 http://ftp.pdbj.org/pub/emdb/structures/EMD-12963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12963 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12963 | HTTPS FTP |
-Validation report
| Summary document |  emd_12963_validation.pdf.gz emd_12963_validation.pdf.gz | 663.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12963_full_validation.pdf.gz emd_12963_full_validation.pdf.gz | 662.9 KB | Display | |
| Data in XML |  emd_12963_validation.xml.gz emd_12963_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_12963_validation.cif.gz emd_12963_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12963 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12963 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12963 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12963 | HTTPS FTP |
-Related structure data
| Related structure data |  7okoMC  7oknC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12963.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12963.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The structure of the outer-membrane core complex (outer ring) from a conjugative type IV secretion system | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Outer-membrane core complex (outer ring)
| Entire | Name: Outer-membrane core complex (outer ring) |
|---|---|
| Components |
|
-Supramolecule #1: Outer-membrane core complex (outer ring)
| Supramolecule | Name: Outer-membrane core complex (outer ring) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Salmonella enterica (bacteria) Salmonella enterica (bacteria) |
-Macromolecule #1: Type IV conjugative transfer system lipoprotein TraV
| Macromolecule | Name: Type IV conjugative transfer system lipoprotein TraV / type: protein_or_peptide / ID: 1 / Number of copies: 26 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica (bacteria) Salmonella enterica (bacteria) |
| Molecular weight | Theoretical: 20.92865 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKKITLLLAG SALLLSGCAG VKSSFDCDAT TSDTCMTMTK ANQLARDKAA KQAGKPAAGG LPSLVNLPAT SAVEVPSASR SAVTPPSGT RTVSTTPPVS AGTSAGVNTN TTTSTLTPRP VAGTPVTTTP SSVAYRPVVS VVTPTPSCQN VRCDNPGTVH P QRSRDQIA ...String: MKKITLLLAG SALLLSGCAG VKSSFDCDAT TSDTCMTMTK ANQLARDKAA KQAGKPAAGG LPSLVNLPAT SAVEVPSASR SAVTPPSGT RTVSTTPPVS AGTSAGVNTN TTTSTLTPRP VAGTPVTTTP SSVAYRPVVS VVTPTPSCQN VRCDNPGTVH P QRSRDQIA TVWIAPWVDS DNAFHQPGRV SFVVSPADWV LPARVN UniProtKB: Type IV conjugative transfer system lipoprotein TraV |
-Macromolecule #2: TraB
| Macromolecule | Name: TraB / type: protein_or_peptide / ID: 2 / Number of copies: 13 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica (bacteria) Salmonella enterica (bacteria) |
| Molecular weight | Theoretical: 1.128233 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PGMMDSQEFS |
-Macromolecule #3: Type-F conjugative transfer system secretin TraK
| Macromolecule | Name: Type-F conjugative transfer system secretin TraK / type: protein_or_peptide / ID: 3 / Number of copies: 26 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. salamae serovar 58:l,z13,z28:z6 (bacteria) Salmonella enterica subsp. salamae serovar 58:l,z13,z28:z6 (bacteria) |
| Molecular weight | Theoretical: 23.312551 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AQSPATISLP QGGQFRLSIS NTDPNMIFIP GDKVTAITAP GGMLADKRLT TAGGVLFTSV ATRTFTIFVE TALGQTFSVV ATPVKGEGR VYRLMSAEPP SRPETRKWET AQAYEKLLIS LNRAVLTGDI PDGYGEVKPL SDGIRLPGGF SVTPLKAWAG D QLRADRYE ...String: AQSPATISLP QGGQFRLSIS NTDPNMIFIP GDKVTAITAP GGMLADKRLT TAGGVLFTSV ATRTFTIFVE TALGQTFSVV ATPVKGEGR VYRLMSAEPP SRPETRKWET AQAYEKLLIS LNRAVLTGDI PDGYGEVKPL SDGIRLPGGF SVTPLKAWAG D QLRADRYE LRNANTWGVA LREQDFWKPG VRAVMFDNNA QTLMGGGRMT VTVIRGNG UniProtKB: Type-F conjugative transfer system secretin TraK |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)