[English] 日本語
 Yorodumi
Yorodumi- EMDB-12591: Cryo-EM structure of S.cerevisiae native alcohol dehydrogenase 1 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of S.cerevisiae native alcohol dehydrogenase 1 (ADH1) in its tetrameric apo state | |||||||||
 Map data Map data | Local sharpen cryo-EM map of apo ADH1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ADH1 / tetramer / S.cerevisiae / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationmethylglyoxal reductase (NADH) / octanol dehydrogenase (NAD+) activity / methylglyoxal reductase (NADH) activity / pyruvate fermentation to ethanol / amino acid catabolic process to alcohol via Ehrlich pathway / butanol dehydrogenase (NAD+) activity / ethanol dehydrogenase (NAD+) activity / melatonin binding / alcohol dehydrogenase (NAD+) activity / allyl-alcohol dehydrogenase ...methylglyoxal reductase (NADH) / octanol dehydrogenase (NAD+) activity / methylglyoxal reductase (NADH) activity / pyruvate fermentation to ethanol / amino acid catabolic process to alcohol via Ehrlich pathway / butanol dehydrogenase (NAD+) activity / ethanol dehydrogenase (NAD+) activity / melatonin binding / alcohol dehydrogenase (NAD+) activity / allyl-alcohol dehydrogenase / allyl-alcohol dehydrogenase activity / alcohol dehydrogenase / zinc ion binding / identical protein binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
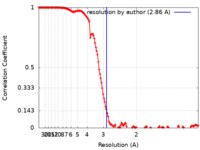
| Method | single particle reconstruction / cryo EM / Resolution: 2.86 Å | |||||||||
 Authors Authors | Nzigou Mandouckou JA / Carroni M | |||||||||
| Funding support |  Sweden, 1 items Sweden, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of S.cerevisiae native alcohol dehydrogenase 1 (ADH1) in its tetrameric apo state Authors: Nzigou Mandouckou JA / Carroni M / Haeggstrom JZ / Thulasingam M | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12591.map.gz emd_12591.map.gz | 214.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12591-v30.xml emd-12591-v30.xml emd-12591.xml emd-12591.xml | 20 KB 20 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12591_fsc.xml emd_12591_fsc.xml | 14 KB | Display |  FSC data file FSC data file |
| Images |  emd_12591.png emd_12591.png | 78 KB | ||
| Masks |  emd_12591_msk_1.map emd_12591_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12591.cif.gz emd-12591.cif.gz | 6.1 KB | ||
| Others |  emd_12591_additional_1.map.gz emd_12591_additional_1.map.gz emd_12591_half_map_1.map.gz emd_12591_half_map_1.map.gz emd_12591_half_map_2.map.gz emd_12591_half_map_2.map.gz | 121.2 MB 226.2 MB 226.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12591 http://ftp.pdbj.org/pub/emdb/structures/EMD-12591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12591 | HTTPS FTP |
-Related structure data
| Related structure data |  7ntmMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12591.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12591.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local sharpen cryo-EM map of apo ADH1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.6072 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_12591_msk_1.map emd_12591_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unfiltered cryo-EM map of apo ADH1
| File | emd_12591_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered cryo-EM map of apo ADH1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A of cryo-EM map of apo ADH1
| File | emd_12591_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A of cryo-EM map of apo ADH1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B of cryo-EM map of apo ADH1
| File | emd_12591_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B of cryo-EM map of apo ADH1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tetrameric structure of native ADH1 from S. cereviciae in its apo...
| Entire | Name: Tetrameric structure of native ADH1 from S. cereviciae in its apo state. |
|---|---|
| Components |
|
-Supramolecule #1: Tetrameric structure of native ADH1 from S. cereviciae in its apo...
| Supramolecule | Name: Tetrameric structure of native ADH1 from S. cereviciae in its apo state. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Stable homotetramer, composed of four monomers labelled as A, B, C and D. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 147 KDa |
-Macromolecule #1: Alcohol dehydrogenase 1
| Macromolecule | Name: Alcohol dehydrogenase 1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: alcohol dehydrogenase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 36.759906 KDa |
| Sequence | String: SIPETQKGVI FYESHGKLEY KDIPVPKPKA NELLINVKYS GVCHTDLHAW HGDWPLPVKL PLVGGHEGAG VVVGMGENVK GWKIGDYAG IKWLNGSCMA CEYCELGNES NCPHADLSGY THDGSFQQYA TADAVQAAHI PQGTDLAQVA PILCAGITVY K ALKSANLM ...String: SIPETQKGVI FYESHGKLEY KDIPVPKPKA NELLINVKYS GVCHTDLHAW HGDWPLPVKL PLVGGHEGAG VVVGMGENVK GWKIGDYAG IKWLNGSCMA CEYCELGNES NCPHADLSGY THDGSFQQYA TADAVQAAHI PQGTDLAQVA PILCAGITVY K ALKSANLM AGHWVAISGA AGGLGSLAVQ YAKAMGYRVL GIDGGEGKEE LFRSIGGEVF IDFTKEKDIV GAVLKATDGG AH GVINVSV SEAAIEASTR YVRANGTTVL VGMPAGAKCC SDVFNQVVKS ISIVGSYVGN RADTREALDF FARGLVKSPI KVV GLSTLP EIYEKMEKGQ IVGRYVVDTS K UniProtKB: Alcohol dehydrogenase 1 |
-Macromolecule #2: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 2 / Number of copies: 8 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Alignment procedure | Coma free - Residual tilt: 130.0 mrad |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 14964 / Average exposure time: 2.5 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 1-347 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Model building and refinement were done using using CCP-EM software suite (Burnley et al, 2017). The X-ray structure of yeast ADH1 (PDB: 5ENV) was used as a starting model for model building. This model was docked into our Cryo-EM map using MolRep (Brown et al, 2014). The resulting starting model was manually adjusted in Coot (Emsley et al, 2010). REFMAC 5 (Brown et al, 2014) used for structure model refinement. |
| Refinement | Space: REAL / Protocol: BACKBONE TRACE / Overall B value: 123 |
| Output model |  PDB-7ntm: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)