+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12063 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Whole MiniTRAPPIII | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Rab1 / GEFs / Golgi / TRAPP complexes / EXOCYTOSIS | |||||||||
| Function / homology |  Function and homology information Function and homology informationCOPII-mediated vesicle transport / RAB GEFs exchange GTP for GDP on RABs / TRAPPI protein complex / TRAPPII protein complex / TRAPPIII protein complex / TRAPP complex / cis-Golgi network membrane / Golgi vesicle transport / Neutrophil degranulation / cis-Golgi network ...COPII-mediated vesicle transport / RAB GEFs exchange GTP for GDP on RABs / TRAPPI protein complex / TRAPPII protein complex / TRAPPIII protein complex / TRAPP complex / cis-Golgi network membrane / Golgi vesicle transport / Neutrophil degranulation / cis-Golgi network / intra-Golgi vesicle-mediated transport / protein secretion / endoplasmic reticulum to Golgi vesicle-mediated transport / long-term memory / intracellular transport / vesicle-mediated transport / trans-Golgi network / nervous system development / spermatogenesis / perinuclear region of cytoplasm / endoplasmic reticulum / Golgi apparatus / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
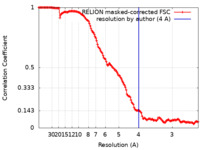
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Galindo A / Munro S | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2021 Journal: EMBO J / Year: 2021Title: Cryo-EM structure of metazoan TRAPPIII, the multi-subunit complex that activates the GTPase Rab1. Authors: Antonio Galindo / Vicente J Planelles-Herrero / Gianluca Degliesposti / Sean Munro /  Abstract: The TRAPP complexes are nucleotide exchange factors that play essential roles in membrane traffic and autophagy. TRAPPII activates Rab11, and TRAPPIII activates Rab1, with the two complexes sharing a ...The TRAPP complexes are nucleotide exchange factors that play essential roles in membrane traffic and autophagy. TRAPPII activates Rab11, and TRAPPIII activates Rab1, with the two complexes sharing a core of small subunits that affect nucleotide exchange but being distinguished by specific large subunits that are essential for activity in vivo. Crystal structures of core subunits have revealed the mechanism of Rab activation, but how the core and the large subunits assemble to form the complexes is unknown. We report a cryo-EM structure of the entire Drosophila TRAPPIII complex. The TRAPPIII-specific subunits TRAPPC8 and TRAPPC11 hold the catalytic core like a pair of tongs, with TRAPPC12 and TRAPPC13 positioned at the joint between them. TRAPPC2 and TRAPPC2L link the core to the two large arms, with the interfaces containing residues affected by disease-causing mutations. The TRAPPC8 arm is positioned such that it would contact Rab1 that is bound to the core, indicating how the arm could determine the specificity of the complex. A lower resolution structure of TRAPPII shows a similar architecture and suggests that the TRAPP complexes evolved from a single ur-TRAPP. | |||||||||
| History |
|
- Structure visualization
Structure visualization
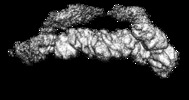
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12063.map.gz emd_12063.map.gz | 228.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12063-v30.xml emd-12063-v30.xml emd-12063.xml emd-12063.xml | 23.5 KB 23.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12063_fsc.xml emd_12063_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_12063.png emd_12063.png | 247.2 KB | ||
| Masks |  emd_12063_msk_1.map emd_12063_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12063.cif.gz emd-12063.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12063 http://ftp.pdbj.org/pub/emdb/structures/EMD-12063 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12063 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12063 | HTTPS FTP |
-Validation report
| Summary document |  emd_12063_validation.pdf.gz emd_12063_validation.pdf.gz | 637.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12063_full_validation.pdf.gz emd_12063_full_validation.pdf.gz | 637.5 KB | Display | |
| Data in XML |  emd_12063_validation.xml.gz emd_12063_validation.xml.gz | 13.5 KB | Display | |
| Data in CIF |  emd_12063_validation.cif.gz emd_12063_validation.cif.gz | 18.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12063 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12063 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12063 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12063 | HTTPS FTP |
-Related structure data
| Related structure data |  7b70MC  7b6dC  7b6eC  7b6hC  7b6rC  7b6xC  7b6yC  7b6zC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12063.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12063.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.2564 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12063_msk_1.map emd_12063_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : MiniTRAPPIII: TRAPPIII complex without the C12 and C13 specific s...
+Supramolecule #1: MiniTRAPPIII: TRAPPIII complex without the C12 and C13 specific s...
+Macromolecule #1: Trafficking protein particle complex subunit
+Macromolecule #2: GEO08327p1
+Macromolecule #3: Trafficking protein particle complex subunit
+Macromolecule #4: Trafficking protein particle complex subunit
+Macromolecule #5: Probable trafficking protein particle complex subunit 2
+Macromolecule #6: Trafficking protein particle complex subunit 5
+Macromolecule #7: TRAPPC2L
+Macromolecule #8: FI18195p1
+Macromolecule #9: Trafficking protein particle complex subunit 11
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.001 kPa | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 285.15 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 3443 / Average exposure time: 0.3 sec. / Average electron dose: 45.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z
Z Y
Y X
X