+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11985 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | TRPC4 in complex with Calmodulin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ION CHANNEL / TRPC4 / COMPLEX / MEMBRANE PROTEIN / CALMODULIN / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationCaMK IV-mediated phosphorylation of CREB / Cam-PDE 1 activation / CREB1 phosphorylation through the activation of CaMKII/CaMKK/CaMKIV cascasde / Glycogen breakdown (glycogenolysis) / Activation of RAC1 downstream of NMDARs / Sodium/Calcium exchangers / Activation of Ca-permeable Kainate Receptor / Synthesis of IP3 and IP4 in the cytosol / CLEC7A (Dectin-1) induces NFAT activation / RHO GTPases activate PAKs ...CaMK IV-mediated phosphorylation of CREB / Cam-PDE 1 activation / CREB1 phosphorylation through the activation of CaMKII/CaMKK/CaMKIV cascasde / Glycogen breakdown (glycogenolysis) / Activation of RAC1 downstream of NMDARs / Sodium/Calcium exchangers / Activation of Ca-permeable Kainate Receptor / Synthesis of IP3 and IP4 in the cytosol / CLEC7A (Dectin-1) induces NFAT activation / RHO GTPases activate PAKs / Calmodulin induced events / Inactivation, recovery and regulation of the phototransduction cascade / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / eNOS activation / Reduction of cytosolic Ca++ levels / Unblocking of NMDA receptors, glutamate binding and activation / Calcineurin activates NFAT / Ion transport by P-type ATPases / Protein methylation / RAF activation / VEGFR2 mediated vascular permeability / RAS processing / Ca2+ pathway / RHO GTPases activate IQGAPs / Extra-nuclear estrogen signaling / FCERI mediated Ca+2 mobilization / RAF/MAP kinase cascade / PKA activation / store-operated calcium channel activity / Smooth Muscle Contraction / Platelet degranulation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Stimuli-sensing channels / inositol 1,4,5 trisphosphate binding / establishment of protein localization to mitochondrial membrane / Ion homeostasis / type 3 metabotropic glutamate receptor binding / cation channel complex / response to corticosterone / negative regulation of ryanodine-sensitive calcium-release channel activity / organelle localization by membrane tethering / mitochondrion-endoplasmic reticulum membrane tethering / autophagosome membrane docking / regulation of cardiac muscle cell action potential / presynaptic endocytosis / nitric-oxide synthase binding / regulation of synaptic vesicle exocytosis / calcineurin-mediated signaling / adenylate cyclase binding / regulation of ryanodine-sensitive calcium-release channel activity / protein phosphatase activator activity / monoatomic cation transport / catalytic complex / detection of calcium ion / regulation of synaptic vesicle endocytosis / regulation of cardiac muscle contraction / regulation of cytosolic calcium ion concentration / postsynaptic cytosol / monoatomic cation channel activity / cellular response to interferon-beta / phosphatidylinositol 3-kinase binding / calcium channel inhibitor activity / presynaptic cytosol / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / titin binding / sperm midpiece / voltage-gated potassium channel complex / calcium channel complex / regulation of heart rate / calyx of Held / response to amphetamine / adenylate cyclase activator activity / sarcomere / nitric-oxide synthase regulator activity / protein serine/threonine kinase activator activity / regulation of cytokinesis / spindle microtubule / positive regulation of receptor signaling pathway via JAK-STAT / mitochondrial membrane / calcium ion transmembrane transport / cellular response to type II interferon / G2/M transition of mitotic cell cycle / Schaffer collateral - CA1 synapse / spindle pole / calcium-dependent protein binding / synaptic vesicle membrane / myelin sheath / growth cone / transmembrane transporter binding / protein domain specific binding / calcium ion binding / centrosome / protein kinase binding / nucleoplasm / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Vinayagam D / Quentin D | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Structural basis of TRPC4 regulation by calmodulin and pharmacological agents. Authors: Deivanayagabarathy Vinayagam / Dennis Quentin / Jing Yu-Strzelczyk / Oleg Sitsel / Felipe Merino / Markus Stabrin / Oliver Hofnagel / Maolin Yu / Mark W Ledeboer / Georg Nagel / Goran ...Authors: Deivanayagabarathy Vinayagam / Dennis Quentin / Jing Yu-Strzelczyk / Oleg Sitsel / Felipe Merino / Markus Stabrin / Oliver Hofnagel / Maolin Yu / Mark W Ledeboer / Georg Nagel / Goran Malojcic / Stefan Raunser /   Abstract: Canonical transient receptor potential channels (TRPC) are involved in receptor-operated and/or store-operated Ca signaling. Inhibition of TRPCs by small molecules was shown to be promising in ...Canonical transient receptor potential channels (TRPC) are involved in receptor-operated and/or store-operated Ca signaling. Inhibition of TRPCs by small molecules was shown to be promising in treating renal diseases. In cells, the channels are regulated by calmodulin (CaM). Molecular details of both CaM and drug binding have remained elusive so far. Here, we report structures of TRPC4 in complex with three pyridazinone-based inhibitors and CaM. The structures reveal that all the inhibitors bind to the same cavity of the voltage-sensing-like domain and allow us to describe how structural changes from the ligand-binding site can be transmitted to the central ion-conducting pore of TRPC4. CaM binds to the rib helix of TRPC4, which results in the ordering of a previously disordered region, fixing the channel in its closed conformation. This represents a novel CaM-induced regulatory mechanism of canonical TRP channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11985.map.gz emd_11985.map.gz | 10.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11985-v30.xml emd-11985-v30.xml emd-11985.xml emd-11985.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11985.png emd_11985.png | 239.7 KB | ||
| Filedesc metadata |  emd-11985.cif.gz emd-11985.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11985 http://ftp.pdbj.org/pub/emdb/structures/EMD-11985 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11985 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11985 | HTTPS FTP |
-Validation report
| Summary document |  emd_11985_validation.pdf.gz emd_11985_validation.pdf.gz | 419.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_11985_full_validation.pdf.gz emd_11985_full_validation.pdf.gz | 419.3 KB | Display | |
| Data in XML |  emd_11985_validation.xml.gz emd_11985_validation.xml.gz | 6.2 KB | Display | |
| Data in CIF |  emd_11985_validation.cif.gz emd_11985_validation.cif.gz | 7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11985 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11985 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11985 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11985 | HTTPS FTP |
-Related structure data
| Related structure data |  7b1gMC  7b05C  7b0jC  7b0sC  7b16C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11985.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11985.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : TRPC4 membrane protein in complex with calmodulin
| Entire | Name: TRPC4 membrane protein in complex with calmodulin |
|---|---|
| Components |
|
-Supramolecule #1: TRPC4 membrane protein in complex with calmodulin
| Supramolecule | Name: TRPC4 membrane protein in complex with calmodulin / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 / Details: TRPC4 was solubilised in LMNG detergent |
|---|---|
| Molecular weight | Theoretical: 126 KDa |
-Supramolecule #2: Transient receptor potential cation channel subfamily c member 4a
| Supramolecule | Name: Transient receptor potential cation channel subfamily c member 4a type: organelle_or_cellular_component / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Calmodulin-1
| Supramolecule | Name: Calmodulin-1 / type: organelle_or_cellular_component / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential cation channel subfamily c member 4a
| Macromolecule | Name: Transient receptor potential cation channel subfamily c member 4a type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 105.204641 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSQLYFRRTD NSSYRDRIPL RIVRAESELS TQEKSYLSAV EKGDYASVKL ALEEAEIYFK ININCIDPLG RTALLIAIEN ENLEIIELL LSFNVYVGDA LLHAIRKEVV GAVELLLNHK KPSGEKQVPP ILLDKQFSDF TPDITPIILA AHTNNYEIIK M LVQKGVSV ...String: GSQLYFRRTD NSSYRDRIPL RIVRAESELS TQEKSYLSAV EKGDYASVKL ALEEAEIYFK ININCIDPLG RTALLIAIEN ENLEIIELL LSFNVYVGDA LLHAIRKEVV GAVELLLNHK KPSGEKQVPP ILLDKQFSDF TPDITPIILA AHTNNYEIIK M LVQKGVSV PQPHEVRCNC VECVSSSDVD SLRHSRSRLN IYKALASPSL IALSSEDPFL TAFQLSWELQ ELSKVENEFK AE YEELSHQ CKHFAKDLLD QTRSSRELEL ILNFRDDMNL LQDEANNELA RLKLAIKYRQ KEFVAQPNCQ QLLASRWYDE FPG WRRRHW AGKLITCVFI GLMFPLLSLC YLVAPKSRYG LFIRKPFIKF ICHTASYLTF LFLLLLASQH IVSNNPDRQG PKPT TVEWM ILPWVLGFIW TEIKQMWDGG FQDYIHDWWN LMDFVMNSLY LATISLKIVA YVKYSGCKPR DTWEMWHPTL VAEAV FAIA NIFSSLRLIS LFTANSHLGP LQISLGRMLL DILKFLFIYC LVLLAFANGL NQLYFYYENS EGMTCKGIRC ERQNNA FST LFETLQSLFW SIFGLISLYV TNVKADHKFT EFVGATMFGT YNVISLVVLL NMLIAMMNNS YQHIADHADI EWKFART KL WMSYFEEGGT LPPPFNIIPS PKSICYLITW IKVHVFKRRS KRTETFGTLG RRAAENVRLN HQYQEVLRNL VKRYVAAM I RDAKTEEGLT EENFKELKQD ISSFRYEVIG MMKGNRKSTR ANKSDTSASD VSHPEGSLQY SSALKQNSKL HLYDVTTAL QQQNSEEAKA SLGCLANGSA VVLTEPILKD KARSDFPKDF TDFGLFPKKQ NPNKIYSLAE EATESDPDIL DWGKEDKPLA GKVEQDVNE SKCLMEEDER VLEEQEMEHI ASSHEH UniProtKB: Short transient receptor potential channel 4b |
-Macromolecule #2: Calmodulin-1
| Macromolecule | Name: Calmodulin-1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 16.852545 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MADQLTEEQI AEFKEAFSLF DKDGDGTITT KELGTVMRSL GQNPTEAELQ DMINEVDADG NGTIDFPEFL TMMARKMKDT DSEEEIREA FRVFDKDGNG YISAAELRHV MTNLGEKLTD EEVDEMIREA DIDGDGQVNY EEFVQMMTAK UniProtKB: Calmodulin-1 |
-Macromolecule #3: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 3 / Number of copies: 4 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #4: (2R)-3-(phosphonooxy)propane-1,2-diyl dihexanoate
| Macromolecule | Name: (2R)-3-(phosphonooxy)propane-1,2-diyl dihexanoate / type: ligand / ID: 4 / Number of copies: 4 / Formula: 44E |
|---|---|
| Molecular weight | Theoretical: 368.36 Da |
| Chemical component information | 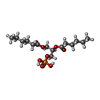 ChemComp-44E: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 160.0 K / Max: 190.0 K |
| Specialist optics | Spherical aberration corrector: Cs corrector first generation Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-80 / Number grids imaged: 1 / Number real images: 7972 / Average exposure time: 10.0 sec. / Average electron dose: 88.52 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 59000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.001 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 58.04 / Target criteria: Correlation coefficient | ||||||
| Output model |  PDB-7b1g: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)























