[English] 日本語
 Yorodumi
Yorodumi- EMDB-1197: A mechanical explanation of RNA pseudoknot function in programmed... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1197 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. | |||||||||
 Map data Map data | 3D reconstruction of IBV pseudoknot-stalled rabbit reticulocyte ribosome containing tRNA, eEF2, pseudoknot and both ribosomal subunits. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Infectious bronchitis virus / Infectious bronchitis virus /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 16.2 Å | |||||||||
 Authors Authors | Namy O / Moran SJ / Stuart DI / Gilbert RJC / Brierley I | |||||||||
 Citation Citation |  Journal: Nature / Year: 2006 Journal: Nature / Year: 2006Title: A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. Authors: Olivier Namy / Stephen J Moran / David I Stuart / Robert J C Gilbert / Ian Brierley /  Abstract: The triplet-based genetic code requires that translating ribosomes maintain the reading frame of a messenger RNA faithfully to ensure correct protein synthesis. However, in programmed -1 ribosomal ...The triplet-based genetic code requires that translating ribosomes maintain the reading frame of a messenger RNA faithfully to ensure correct protein synthesis. However, in programmed -1 ribosomal frameshifting, a specific subversion of frame maintenance takes place, wherein the ribosome is forced to shift one nucleotide backwards into an overlapping reading frame and to translate an entirely new sequence of amino acids. This process is indispensable in the replication of numerous viral pathogens, including HIV and the coronavirus associated with severe acute respiratory syndrome, and is also exploited in the expression of several cellular genes. Frameshifting is promoted by an mRNA signal composed of two essential elements: a heptanucleotide 'slippery' sequence and an adjacent mRNA secondary structure, most often an mRNA pseudoknot. How these components operate together to manipulate the ribosome is unknown. Here we describe the observation of a ribosome-mRNA pseudoknot complex that is stalled in the process of -1 frameshifting. Cryoelectron microscopic imaging of purified mammalian 80S ribosomes from rabbit reticulocytes paused at a coronavirus pseudoknot reveals an intermediate of the frameshifting process. From this it can be seen how the pseudoknot interacts with the ribosome to block the mRNA entrance channel, compromising the translocation process and leading to a spring-like deformation of the P-site transfer RNA. In addition, we identify movements of the likely eukaryotic ribosomal helicase and confirm a direct interaction between the translocase eEF2 and the P-site tRNA. Together, the structural changes provide a mechanical explanation of how the pseudoknot manipulates the ribosome into a different reading frame. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1197.map.gz emd_1197.map.gz | 494.9 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1197-v30.xml emd-1197-v30.xml emd-1197.xml emd-1197.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| Images |  1197.gif 1197.gif | 45.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1197 http://ftp.pdbj.org/pub/emdb/structures/EMD-1197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1197 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1197.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1197.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of IBV pseudoknot-stalled rabbit reticulocyte ribosome containing tRNA, eEF2, pseudoknot and both ribosomal subunits. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.33 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
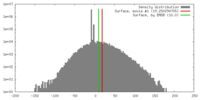
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Rabbit reticuloyte ribosome stalled on IBV mRNA containing pseudo...
| Entire | Name: Rabbit reticuloyte ribosome stalled on IBV mRNA containing pseudoknot with tRNA and eEF2 |
|---|---|
| Components |
|
-Supramolecule #1000: Rabbit reticuloyte ribosome stalled on IBV mRNA containing pseudo...
| Supramolecule | Name: Rabbit reticuloyte ribosome stalled on IBV mRNA containing pseudoknot with tRNA and eEF2 type: sample / ID: 1000 Oligomeric state: One each of 40S, 60S, mRNA, eEF2 and P-site tRNA Number unique components: 5 |
|---|---|
| Molecular weight | Experimental: 3.5 MDa / Theoretical: 3.5 MDa |
-Supramolecule #1: Small subunit
| Supramolecule | Name: Small subunit / type: complex / ID: 1 / Name.synonym: 40S / Ribosome-details: ribosome-eukaryote: SSU 40S |
|---|
-Supramolecule #2: Large subunit
| Supramolecule | Name: Large subunit / type: complex / ID: 2 / Name.synonym: 60S / Ribosome-details: ribosome-eukaryote: LSU 60S |
|---|
-Macromolecule #1: Message with pseudoknot
| Macromolecule | Name: Message with pseudoknot / type: rna / ID: 1 / Name.synonym: mRNA / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:  Infectious bronchitis virus / synonym: IBV Infectious bronchitis virus / synonym: IBV |
-Macromolecule #3: P-site transfer RNA
| Macromolecule | Name: P-site transfer RNA / type: rna / ID: 3 / Name.synonym: tRNA / Classification: OTHER / Structure: DOUBLE HELIX / Synthetic?: No |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #2: Elongation translocase
| Macromolecule | Name: Elongation translocase / type: protein_or_peptide / ID: 2 / Name.synonym: eEF2 / Number of copies: 1 / Oligomeric state: Monomer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Grid | Details: 300 mesh gold w/ lacey carbon |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Temperature | Average: 100 K |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 3.33 µm / Number real images: 31 / Average electron dose: 2 e/Å2 / Od range: 5 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 7.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | The particles were selected in a semi-automated fashion using BOXER (EMAN suite) |
|---|---|
| CTF correction | Details: By micrograph |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 16.2 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER and GAP Details: Deposited map is composite of computationally-separated subunits and bound co-factors from the final map. Number images used: 17672 |
| Final angle assignment | Details: SPIDER |
-Atomic model buiding 1
| Software | Name: URO |
|---|---|
| Details | Protocol: Rigid body. Domains manually docked in O and refined in URO |
| Refinement | Protocol: RIGID BODY FIT / Target criteria: CC |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)