[English] 日本語
 Yorodumi
Yorodumi- EMDB-1166: Cryo-EM reconstruction of dengue virus in complex with the carboh... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1166 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. | |||||||||
 Map data Map data | EM map of dengue virus in complex with CRD domain of DC-SIGN | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationB cell adhesion / cell-cell recognition / intracellular transport of virus / peptide antigen transport / Butyrophilin (BTN) family interactions / positive regulation of viral life cycle / virion binding / heterophilic cell-cell adhesion / leukocyte cell-cell adhesion / antigen processing and presentation ...B cell adhesion / cell-cell recognition / intracellular transport of virus / peptide antigen transport / Butyrophilin (BTN) family interactions / positive regulation of viral life cycle / virion binding / heterophilic cell-cell adhesion / leukocyte cell-cell adhesion / antigen processing and presentation / pattern recognition receptor activity / RSV-host interactions / D-mannose binding / regulation of T cell proliferation / host cell mitochondrion / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of host TYK2 activity / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT2 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / CD209 (DC-SIGN) signaling / positive regulation of T cell proliferation / viral genome replication / peptide antigen binding / endocytosis / ribonucleoside triphosphate phosphatase activity / viral capsid / double-stranded RNA binding / host cell / channel activity / carbohydrate binding / virus receptor activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / molecular adaptor activity / adaptive immune response / methyltransferase cap1 activity / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / RNA helicase activity / protein dimerization activity / intracellular signal transduction / immune response / host cell perinuclear region of cytoplasm / host cell endoplasmic reticulum membrane / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / serine-type endopeptidase activity / external side of plasma membrane / symbiont-mediated activation of host autophagy / innate immune response / viral RNA genome replication / RNA-directed RNA polymerase activity / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / lipid binding / virion attachment to host cell / host cell nucleus / virion membrane / structural molecule activity / cell surface / proteolysis / extracellular region / ATP binding / metal ion binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Dengue-2 Homo sapiens (human) / Dengue-2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 25.0 Å | |||||||||
 Authors Authors | Pokidysheva E / Zhang Y / Battisti AJ / Bator-Kelly CM / Chipman PR / Xiao C / Gregorio GG / Hendrickson WA / Kuhn RJ / Rossmann MG | |||||||||
 Citation Citation |  Journal: Cell / Year: 2006 Journal: Cell / Year: 2006Title: Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Authors: Elena Pokidysheva / Ying Zhang / Anthony J Battisti / Carol M Bator-Kelly / Paul R Chipman / Chuan Xiao / G Glenn Gregorio / Wayne A Hendrickson / Richard J Kuhn / Michael G Rossmann /  Abstract: Dengue virus (DENV) is a significant human pathogen that causes millions of infections and results in about 24,000 deaths each year. Dendritic cell-specific ICAM3 grabbing nonintegrin (DC-SIGN), ...Dengue virus (DENV) is a significant human pathogen that causes millions of infections and results in about 24,000 deaths each year. Dendritic cell-specific ICAM3 grabbing nonintegrin (DC-SIGN), abundant in immature dendritic cells, was previously reported as being an ancillary receptor interacting with the surface of DENV. The structure of DENV in complex with the carbohydrate recognition domain (CRD) of DC-SIGN was determined by cryo-electron microscopy at 25 A resolution. One CRD monomer was found to bind to two glycosylation sites at Asn67 of two neighboring glycoproteins in each icosahedral asymmetric unit, leaving the third Asn67 residue vacant. The vacancy at the third Asn67 site is a result of the nonequivalence of the glycoprotein environments, leaving space for the primary receptor binding to domain III of E. The use of carbohydrate moieties for receptor binding sites suggests a mechanism for avoiding immune surveillance. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1166.map.gz emd_1166.map.gz | 4.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1166-v30.xml emd-1166-v30.xml emd-1166.xml emd-1166.xml | 11.5 KB 11.5 KB | Display Display |  EMDB header EMDB header |
| Images |  1166.gif 1166.gif | 12 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1166 http://ftp.pdbj.org/pub/emdb/structures/EMD-1166 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1166 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1166 | HTTPS FTP |
-Related structure data
| Related structure data |  2b6bMC  1167C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1166.map.gz / Format: CCP4 / Size: 13.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1166.map.gz / Format: CCP4 / Size: 13.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
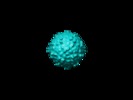
| Annotation | EM map of dengue virus in complex with CRD domain of DC-SIGN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Dengue virus complexed with CRD domain of DC-SIGN
| Entire | Name: Dengue virus complexed with CRD domain of DC-SIGN |
|---|---|
| Components |
|
-Supramolecule #1000: Dengue virus complexed with CRD domain of DC-SIGN
| Supramolecule | Name: Dengue virus complexed with CRD domain of DC-SIGN / type: sample / ID: 1000 / Details: Calcium should be present in the sample. / Oligomeric state: one monomer of CRD binds virus ico / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 12 MDa |
-Supramolecule #1: Dengue-2
| Supramolecule | Name: Dengue-2 / type: virus / ID: 1 / Sci species name: Dengue-2 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
| Molecular weight | Experimental: 11 MDa / Theoretical: 11 MDa |
| Virus shell | Shell ID: 1 / Name: outer shell, T is not applicable / Diameter: 500 Å / T number (triangulation number): 3 |
-Macromolecule #1: CRD domain of DC-SIGN
| Macromolecule | Name: CRD domain of DC-SIGN / type: protein_or_peptide / ID: 1 Details: expressed insoluble in the inclusion bodies. Refolded. Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human / Tissue: dendritic cells / Cell: bacteria BL21-DE3 / Location in cell: cell membrane, extracellular matrix Homo sapiens (human) / synonym: Human / Tissue: dendritic cells / Cell: bacteria BL21-DE3 / Location in cell: cell membrane, extracellular matrix |
| Molecular weight | Experimental: 18 KDa / Theoretical: 18 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 50mM Tris 50mM NaCl 0.5 mM EDTA, 5 mM CaCl2, pH 7.5 |
| Grid | Details: 400 mesh copper grid |
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: Guillotine-style plunge freezeing device Method: Small aliquots of sample were applied to 400 mesh copper grids coated with holey carbon film and rapidly frozen by plunging into an ethane slush |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM300FEG/T |
|---|---|
| Temperature | Average: 103 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at 98,000 times magnification |
| Date | Nov 15, 2004 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 45 / Average electron dose: 11.8 e/Å2 / Od range: 1.1 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 33000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 33000 |
| Sample stage | Specimen holder: Cryo / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | the particles were selected manualy |
|---|---|
| CTF correction | Details: each particle |
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 25.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: spider / Number images used: 830 |
| Final angle assignment | Details: SPIDER theta 36.4 degrees, phi 72 degrees |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)





















