[English] 日本語
 Yorodumi
Yorodumi- EMDB-11643: Structure of the split human mitoribosomal large subunit with res... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11643 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the split human mitoribosomal large subunit with rescue factors mtRF-R and MTRES1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of mitochondrial transcription / negative regulation of mitochondrial translation / mitochondrial large ribosomal subunit assembly / negative regulation of ribosome biogenesis / Mitochondrial Fatty Acid Beta-Oxidation / Protein lipoylation / protein lipoylation / positive regulation of mitochondrial translation / Complex I biogenesis / Respiratory electron transport ...regulation of mitochondrial transcription / negative regulation of mitochondrial translation / mitochondrial large ribosomal subunit assembly / negative regulation of ribosome biogenesis / Mitochondrial Fatty Acid Beta-Oxidation / Protein lipoylation / protein lipoylation / positive regulation of mitochondrial translation / Complex I biogenesis / Respiratory electron transport / mitochondrial translational termination / Mitochondrial ribosome-associated quality control / mitochondrial translational elongation / Mitochondrial translation elongation / translation release factor activity, codon nonspecific / Mitochondrial translation initiation / Mitochondrial translation termination / mitochondrial fission / iron-sulfur cluster assembly complex / translation release factor activity / mitochondrial large ribosomal subunit binding / mitochondrial large ribosomal subunit / peptidyl-tRNA hydrolase / mitochondrial [2Fe-2S] assembly complex / mitochondrial ribosome / Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters / mitochondrial small ribosomal subunit / peptidyl-tRNA hydrolase activity / [2Fe-2S] cluster assembly / mitochondrial translation / iron-sulfur cluster assembly / acyl binding / acyl carrier activity / ribosomal large subunit binding / proton motive force-driven mitochondrial ATP synthesis / mitochondrial respiratory chain complex I assembly / mitochondrial electron transport, NADH to ubiquinone / respiratory chain complex I / anatomical structure morphogenesis / RNA processing / Mitochondrial protein degradation / rescue of stalled cytosolic ribosome / fatty acid binding / aerobic respiration / cellular response to leukemia inhibitory factor / ribosomal large subunit biogenesis / mitochondrial membrane / fibrillar center / fatty acid biosynthetic process / cell junction / double-stranded RNA binding / large ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / endonuclease activity / tRNA binding / mitochondrial inner membrane / negative regulation of translation / rRNA binding / nuclear body / structural constituent of ribosome / ribosome / translation / mitochondrial matrix / ribonucleoprotein complex / protein domain specific binding / nucleotide binding / mRNA binding / apoptotic process / calcium ion binding / nucleolus / structural molecule activity / mitochondrion / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  human (human) human (human) | ||||||||||||
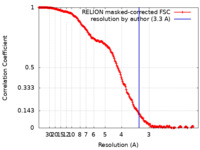
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||
 Authors Authors | Desai N / Yang H / Chandrasekaran V / Kazi R / Minczuk M / Ramakrishnan V | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Elongational stalling activates mitoribosome-associated quality control. Authors: Nirupa Desai / Hanting Yang / Viswanathan Chandrasekaran / Razina Kazi / Michal Minczuk / V Ramakrishnan /  Abstract: The human mitochondrial ribosome (mitoribosome) and associated proteins regulate the synthesis of 13 essential subunits of the oxidative phosphorylation complexes. We report the discovery of a ...The human mitochondrial ribosome (mitoribosome) and associated proteins regulate the synthesis of 13 essential subunits of the oxidative phosphorylation complexes. We report the discovery of a mitoribosome-associated quality control pathway that responds to interruptions during elongation, and we present structures at 3.1- to 3.3-angstrom resolution of mitoribosomal large subunits trapped during ribosome rescue. Release factor homolog C12orf65 (mtRF-R) and RNA binding protein C6orf203 (MTRES1) eject the nascent chain and peptidyl transfer RNA (tRNA), respectively, from stalled ribosomes. Recruitment of mitoribosome biogenesis factors to these quality control intermediates suggests additional roles for these factors during mitoribosome rescue. We also report related cryo-electron microscopy structures (3.7 to 4.4 angstrom resolution) of elongating mitoribosomes bound to tRNAs, nascent polypeptides, the guanosine triphosphatase elongation factors mtEF-Tu and mtEF-G1, and the Oxa1L translocase. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11643.map.gz emd_11643.map.gz | 56.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11643-v30.xml emd-11643-v30.xml emd-11643.xml emd-11643.xml | 73.7 KB 73.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11643_fsc.xml emd_11643_fsc.xml | 18.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_11643.png emd_11643.png | 174.1 KB | ||
| Others |  emd_11643_additional_1.map.gz emd_11643_additional_1.map.gz emd_11643_half_map_1.map.gz emd_11643_half_map_1.map.gz emd_11643_half_map_2.map.gz emd_11643_half_map_2.map.gz | 412.9 MB 414.8 MB 414.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11643 http://ftp.pdbj.org/pub/emdb/structures/EMD-11643 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11643 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11643 | HTTPS FTP |
-Related structure data
| Related structure data |  7a5hMC  7a5fC  7a5gC  7a5iC  7a5jC  7a5kC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11643.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11643.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Refined map
| File | emd_11643_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half2 map
| File | emd_11643_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half2 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half1 map
| File | emd_11643_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half1 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : human mitoribosome
+Supramolecule #1: human mitoribosome
+Macromolecule #1: 39S ribosomal protein L2, mitochondrial
+Macromolecule #2: 39S ribosomal protein L3, mitochondrial
+Macromolecule #3: 39S ribosomal protein L4, mitochondrial
+Macromolecule #4: 39S ribosomal protein L9, mitochondrial
+Macromolecule #5: 39S ribosomal protein L10, mitochondrial
+Macromolecule #6: 39S ribosomal protein L11, mitochondrial
+Macromolecule #7: 39S ribosomal protein L13, mitochondrial
+Macromolecule #8: 39S ribosomal protein L14, mitochondrial
+Macromolecule #9: 39S ribosomal protein L15, mitochondrial
+Macromolecule #10: 39S ribosomal protein L16, mitochondrial
+Macromolecule #11: 39S ribosomal protein L17, mitochondrial
+Macromolecule #12: Mitochondrial ribosomal protein L18, isoform CRA_b
+Macromolecule #13: 39S ribosomal protein L19, mitochondrial
+Macromolecule #14: 39S ribosomal protein L20, mitochondrial
+Macromolecule #15: 39S ribosomal protein L21, mitochondrial
+Macromolecule #16: 39S ribosomal protein L22, mitochondrial
+Macromolecule #17: 39S ribosomal protein L23, mitochondrial
+Macromolecule #18: 39S ribosomal protein L24, mitochondrial
+Macromolecule #19: 39S ribosomal protein L27, mitochondrial
+Macromolecule #20: 39S ribosomal protein L28, mitochondrial
+Macromolecule #21: 39S ribosomal protein L47, mitochondrial
+Macromolecule #22: 39S ribosomal protein L30, mitochondrial
+Macromolecule #23: 39S ribosomal protein L32, mitochondrial
+Macromolecule #24: 39S ribosomal protein L33, mitochondrial
+Macromolecule #25: 39S ribosomal protein L34, mitochondrial
+Macromolecule #26: 39S ribosomal protein L35, mitochondrial
+Macromolecule #27: 39S ribosomal protein L37, mitochondrial
+Macromolecule #28: 39S ribosomal protein L38, mitochondrial
+Macromolecule #29: 39S ribosomal protein L39, mitochondrial
+Macromolecule #30: 39S ribosomal protein L40, mitochondrial
+Macromolecule #31: 39S ribosomal protein L41, mitochondrial
+Macromolecule #32: 39S ribosomal protein L42, mitochondrial
+Macromolecule #33: 39S ribosomal protein L43, mitochondrial
+Macromolecule #34: 39S ribosomal protein L44, mitochondrial
+Macromolecule #35: 39S ribosomal protein L45, mitochondrial
+Macromolecule #36: 39S ribosomal protein L46, mitochondrial
+Macromolecule #37: 39S ribosomal protein L48, mitochondrial
+Macromolecule #38: 39S ribosomal protein L49, mitochondrial
+Macromolecule #39: 39S ribosomal protein L50, mitochondrial
+Macromolecule #40: 39S ribosomal protein L51, mitochondrial
+Macromolecule #41: cDNA FLJ76418, highly similar to Homo sapiens mitochondrial ribos...
+Macromolecule #42: 39S ribosomal protein L53, mitochondrial
+Macromolecule #43: 39S ribosomal protein L54, mitochondrial
+Macromolecule #44: 39S ribosomal protein L55, mitochondrial
+Macromolecule #45: Ribosomal protein 63, mitochondrial
+Macromolecule #46: Peptidyl-tRNA hydrolase ICT1, mitochondrial
+Macromolecule #47: Growth arrest and DNA damage-inducible proteins-interacting protein 1
+Macromolecule #48: 39S ribosomal protein S18a, mitochondrial
+Macromolecule #49: 39S ribosomal protein S30, mitochondrial
+Macromolecule #50: Unknown protein/protein extension
+Macromolecule #51: Mitochondrial assembly of ribosomal large subunit protein 1
+Macromolecule #52: MIEF1 upstream open reading frame protein
+Macromolecule #53: Acyl carrier protein, mitochondrial
+Macromolecule #57: nascent chain
+Macromolecule #58: Probable peptide chain release factor C12orf65, mitochondrial
+Macromolecule #59: Mitochondrial transcription rescue factor 1
+Macromolecule #54: 16S rRNA
+Macromolecule #55: mt-tRNAVal
+Macromolecule #56: mt-tRNA
+Macromolecule #60: MAGNESIUM ION
+Macromolecule #61: ZINC ION
+Macromolecule #62: 4'-PHOSPHOPANTETHEINE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)