[English] 日本語
 Yorodumi
Yorodumi- EMDB-11571: Human C Complex Spliceosome - MultiBody refined BRR2/PRP16 region -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11571 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human C Complex Spliceosome - MultiBody refined BRR2/PRP16 region | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.1 Å | |||||||||
 Authors Authors | Bertram K | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2020 Journal: Mol Cell / Year: 2020Title: Structural Insights into the Roles of Metazoan-Specific Splicing Factors in the Human Step 1 Spliceosome. Authors: Karl Bertram / Leyla El Ayoubi / Olexandr Dybkov / Dmitry E Agafonov / Cindy L Will / Klaus Hartmuth / Henning Urlaub / Berthold Kastner / Holger Stark / Reinhard Lührmann /  Abstract: Human spliceosomes contain numerous proteins absent in yeast, whose functions remain largely unknown. Here we report a 3D cryo-EM structure of the human spliceosomal C complex at 3.4 Å core ...Human spliceosomes contain numerous proteins absent in yeast, whose functions remain largely unknown. Here we report a 3D cryo-EM structure of the human spliceosomal C complex at 3.4 Å core resolution and 4.5-5.7 Å at its periphery, and aided by protein crosslinking we determine its molecular architecture. Our structure provides additional insights into the spliceosome's architecture between the catalytic steps of splicing, and how proteins aid formation of the spliceosome's catalytically active RNP (ribonucleoprotein) conformation. It reveals the spatial organization of the metazoan-specific proteins PPWD1, WDR70, FRG1, and CIR1 in human C complexes, indicating they stabilize functionally important protein domains and RNA structures rearranged/repositioned during the B to C transition. Structural comparisons with human B, C, and P complexes reveal an intricate cascade of RNP rearrangements during splicing catalysis, with intermediate RNP conformations not found in yeast, and additionally elucidate the structural basis for the sequential recruitment of metazoan-specific spliceosomal proteins. | |||||||||
| History |
|
- Structure visualization
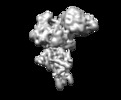
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11571.map.gz emd_11571.map.gz | 166.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11571-v30.xml emd-11571-v30.xml emd-11571.xml emd-11571.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11571.png emd_11571.png | 64.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11571 http://ftp.pdbj.org/pub/emdb/structures/EMD-11571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11571 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11571.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11571.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
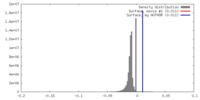
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human C complex Spliceosome - High resolution core
| Entire | Name: Human C complex Spliceosome - High resolution core |
|---|---|
| Components |
|
-Supramolecule #1: Human C complex Spliceosome - High resolution core
| Supramolecule | Name: Human C complex Spliceosome - High resolution core / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#26 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Strain: HeLa S3 Homo sapiens (human) / Strain: HeLa S3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.9 Component:
| ||||||||||||
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK II | ||||||||||||
| Details | Monodisperse particle distribution |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 2 / Number real images: 28279 / Average exposure time: 1.0 sec. / Average electron dose: 120.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -0.003 µm / Nominal defocus min: -0.001 µm / Nominal magnification: 132000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 1751359 |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 69000 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)