[English] 日本語
 Yorodumi
Yorodumi- EMDB-1072: Three-dimensional structures of translating ribosomes by Cryo-EM. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1072 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Three-dimensional structures of translating ribosomes by Cryo-EM. | |||||||||
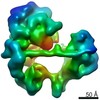
 Map data Map data | 3D reconstruction of E. coli ribosome stalled in translation of Green Fluorescent Protein | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 16.0 Å | |||||||||
 Authors Authors | Gilbert RJC / Fucini P / Connell S / Fuller SD / Nierhaus KH / Robinson CV / Dobson CM / Stuart DI | |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2004 Journal: Mol Cell / Year: 2004Title: Three-dimensional structures of translating ribosomes by Cryo-EM. Authors: Robert J C Gilbert / Paola Fucini / Sean Connell / Stephen D Fuller / Knud H Nierhaus / Carol V Robinson / Christopher M Dobson / David I Stuart /  Abstract: Cryo-electron microscopy and image reconstruction techniques have been used to obtain three-dimensional maps for E. coli ribosomes stalled following translation of three representative proteins. ...Cryo-electron microscopy and image reconstruction techniques have been used to obtain three-dimensional maps for E. coli ribosomes stalled following translation of three representative proteins. Comparisons of these electron density maps, at resolutions of between 13 and 16 A, with that of a nontranslating ribosome pinpoint specific structural differences in stalled ribosomes and identify additional material, including tRNAs and mRNA. In addition, the tunnel through the large subunit, the anticipated exit route of newly synthesized proteins, is partially occluded in all the stalled ribosome structures. This observation suggests that significant segments of the nascent polypeptide chains examined here could be located within an expanded tunnel, perhaps in a rudimentary globular conformation. Such behavior could be an important aspect of the folding of at least some proteins in the cellular environment. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1072.map.gz emd_1072.map.gz | 7.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1072-v30.xml emd-1072-v30.xml emd-1072.xml emd-1072.xml | 12.2 KB 12.2 KB | Display Display |  EMDB header EMDB header |
| Images |  1072.gif 1072.gif | 61.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1072 http://ftp.pdbj.org/pub/emdb/structures/EMD-1072 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1072 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1072 | HTTPS FTP |
-Validation report
| Summary document |  emd_1072_validation.pdf.gz emd_1072_validation.pdf.gz | 236.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1072_full_validation.pdf.gz emd_1072_full_validation.pdf.gz | 235.5 KB | Display | |
| Data in XML |  emd_1072_validation.xml.gz emd_1072_validation.xml.gz | 5.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1072 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1072 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1072 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1072 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1072.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1072.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of E. coli ribosome stalled in translation of Green Fluorescent Protein | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.33 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : E. coli ribosome translating GFP
| Entire | Name: E. coli ribosome translating GFP |
|---|---|
| Components |
|
-Supramolecule #1000: E. coli ribosome translating GFP
| Supramolecule | Name: E. coli ribosome translating GFP / type: sample / ID: 1000 / Details: The sample was monodisperse. Oligomeric state: 30S subunit and 50S subunit, 2 tRNA molecules and 1 nascent protein Number unique components: 5 |
|---|
-Supramolecule #1: E. coli 30S subunit
| Supramolecule | Name: E. coli 30S subunit / type: complex / ID: 1 / Name.synonym: 30S / Recombinant expression: No / Ribosome-details: ribosome-prokaryote: SSU 30S |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #2: E. coli 50S subunit
| Supramolecule | Name: E. coli 50S subunit / type: complex / ID: 2 / Name.synonym: 50S / Recombinant expression: No / Ribosome-details: ribosome-prokaryote: LSU 50S |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: P site tRNA
| Macromolecule | Name: P site tRNA / type: rna / ID: 1 / Details: partial occupancy / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #2: E site tRNA
| Macromolecule | Name: E site tRNA / type: rna / ID: 2 / Details: partial occupancy lower than P site / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #3: Green fluorescent protein
| Macromolecule | Name: Green fluorescent protein / type: protein_or_peptide / ID: 3 / Name.synonym: GFP / Number of copies: 1 / Oligomeric state: Monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism: In vitro transcription.translation system / Recombinant plasmid: pIVEX2.3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.0 mg/mL |
|---|---|
| Buffer | Details: 20 mM HEPES, 150 mM ammonium acetate, 6 mM magnesium acetate, 2 mM spermidine, 0.05 mM spermine and 4 mM 2-mercaptoethanol. Concentration of ribosomes expressed to A260 units. |
| Grid | Details: 300 mesh copper grid with holey carbon film |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 100 K / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: Standard unmodified guillotine plunger |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Temperature | Average: 100 K |
| Date | May 2, 2000 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 8.322 µm / Number real images: 10 / Details: Scanner model:UMAX PowerLook 3000 / Od range: 5 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2 mm / Nominal defocus max: 5.96 µm / Nominal defocus min: 1.805 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| CTF correction | Details: Each negative dataset |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 16.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER, IMAGIC, GAP, CNS, XPLOR Details: Final maps were calculated from 2276 images from a total of 4500 from 10 individual datasets, with scaling in reciprocal space to crystallographic ribosome structures and correction for map ...Details: Final maps were calculated from 2276 images from a total of 4500 from 10 individual datasets, with scaling in reciprocal space to crystallographic ribosome structures and correction for map anisotropy by B-factor weighting of amplitudes in XPLOR with respect to a similarly-treated control inactive ribosome. Selection of particles for inclusion in the final maps was by correlation coefficient with respect to the alignment model, designed to maximise nascent chain occupancy in the selected images. Number images used: 2276 |
| Final angle assignment | Details: SPIDER euler |
-Atomic model buiding 1
| Software | Name: GAP |
|---|---|
| Details | Protocol: Rigid body |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: R-factor and correlation coefficient |
 Movie
Movie Controller
Controller













 Y (Sec.)
Y (Sec.) X (Row.)
X (Row.) Z (Col.)
Z (Col.)





















