[English] 日本語
 Yorodumi
Yorodumi- EMDB-10227: Cryo-EM structure of the CMG Fork Protection Complex at a replica... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10227 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the CMG Fork Protection Complex at a replication fork - Conformation 1 | |||||||||
 Map data Map data | Conformation1 CMG-Csm3-Tof1-Mrc1-Ctf4 and a DNA fork | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | protein-DNA complex / replisome / AAA+ helicase / CMG / GINS / fork DNA / MCM / fork protection complex / CIP-box / REPLICATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationestablishment of sister chromatid cohesion / Unwinding of DNA / maintenance of DNA repeat elements / replication fork arrest / Cul8-RING ubiquitin ligase complex / meiotic chromosome segregation / MCM core complex / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / DNA strand elongation involved in mitotic DNA replication ...establishment of sister chromatid cohesion / Unwinding of DNA / maintenance of DNA repeat elements / replication fork arrest / Cul8-RING ubiquitin ligase complex / meiotic chromosome segregation / MCM core complex / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / DNA strand elongation involved in mitotic DNA replication / GINS complex / MCM complex binding / mitotic DNA replication preinitiation complex assembly / nuclear DNA replication / premeiotic DNA replication / replication fork protection complex / pre-replicative complex assembly involved in nuclear cell cycle DNA replication / Activation of the pre-replicative complex / mitotic DNA replication / CMG complex / DNA replication checkpoint signaling / establishment of mitotic sister chromatid cohesion / nuclear pre-replicative complex / DNA replication preinitiation complex / Activation of ATR in response to replication stress / MCM complex / double-strand break repair via break-induced replication / mitotic DNA replication initiation / single-stranded DNA helicase activity / regulation of DNA-templated DNA replication initiation / silent mating-type cassette heterochromatin formation / mitotic sister chromatid cohesion / DNA strand elongation involved in DNA replication / nuclear chromosome / nuclear replication fork / replication fork processing / DNA replication origin binding / DNA replication initiation / subtelomeric heterochromatin formation / DNA helicase activity / meiotic cell cycle / transcription elongation by RNA polymerase II / helicase activity / DNA-templated DNA replication / heterochromatin formation / mitotic cell cycle / nucleosome assembly / single-stranded DNA binding / DNA helicase / DNA replication / chromosome, telomeric region / DNA repair / DNA damage response / chromatin binding / ATP hydrolysis activity / DNA binding / zinc ion binding / nucleoplasm / ATP binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
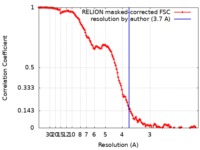
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Baretic D / Jenkyn-Bedford M | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2020 Journal: Mol Cell / Year: 2020Title: Cryo-EM Structure of the Fork Protection Complex Bound to CMG at a Replication Fork. Authors: Domagoj Baretić / Michael Jenkyn-Bedford / Valentina Aria / Giuseppe Cannone / Mark Skehel / Joseph T P Yeeles /  Abstract: The eukaryotic replisome, organized around the Cdc45-MCM-GINS (CMG) helicase, orchestrates chromosome replication. Multiple factors associate directly with CMG, including Ctf4 and the heterotrimeric ...The eukaryotic replisome, organized around the Cdc45-MCM-GINS (CMG) helicase, orchestrates chromosome replication. Multiple factors associate directly with CMG, including Ctf4 and the heterotrimeric fork protection complex (Csm3/Tof1 and Mrc1), which has important roles including aiding normal replication rates and stabilizing stalled forks. How these proteins interface with CMG to execute these functions is poorly understood. Here we present 3 to 3.5 Å resolution electron cryomicroscopy (cryo-EM) structures comprising CMG, Ctf4, and the fork protection complex at a replication fork. The structures provide high-resolution views of CMG-DNA interactions, revealing a mechanism for strand separation, and show Csm3/Tof1 "grip" duplex DNA ahead of CMG via a network of interactions important for efficient replication fork pausing. Although Mrc1 was not resolved in our structures, we determine its topology in the replisome by cross-linking mass spectrometry. Collectively, our work reveals how four highly conserved replisome components collaborate with CMG to facilitate replisome progression and maintain genome stability. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10227.map.gz emd_10227.map.gz | 161.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10227-v30.xml emd-10227-v30.xml emd-10227.xml emd-10227.xml | 46.5 KB 46.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10227_fsc.xml emd_10227_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_10227.png emd_10227.png | 106.5 KB | ||
| Filedesc metadata |  emd-10227.cif.gz emd-10227.cif.gz | 14.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10227 http://ftp.pdbj.org/pub/emdb/structures/EMD-10227 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10227 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10227 | HTTPS FTP |
-Validation report
| Summary document |  emd_10227_validation.pdf.gz emd_10227_validation.pdf.gz | 650.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10227_full_validation.pdf.gz emd_10227_full_validation.pdf.gz | 650.3 KB | Display | |
| Data in XML |  emd_10227_validation.xml.gz emd_10227_validation.xml.gz | 13 KB | Display | |
| Data in CIF |  emd_10227_validation.cif.gz emd_10227_validation.cif.gz | 17.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10227 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10227 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10227 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10227 | HTTPS FTP |
-Related structure data
| Related structure data |  6sklMC  6skoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10227.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10227.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Conformation1 CMG-Csm3-Tof1-Mrc1-Ctf4 and a DNA fork | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.049 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
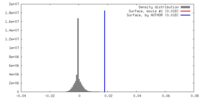
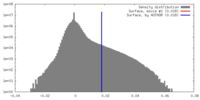
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Conformation 1 of CMG-Csm3-Tof1-Mrc1-Ctf4 with a fork DNA
+Supramolecule #1: Conformation 1 of CMG-Csm3-Tof1-Mrc1-Ctf4 with a fork DNA
+Supramolecule #2: CMG-Csm3-Tof1-Mrc1-Ctf4
+Supramolecule #3: DNA
+Macromolecule #1: DNA replication licensing factor MCM2
+Macromolecule #2: DNA replication licensing factor MCM3
+Macromolecule #3: DNA replication licensing factor MCM4
+Macromolecule #4: Minichromosome maintenance protein 5
+Macromolecule #5: DNA replication licensing factor MCM6
+Macromolecule #6: DNA replication licensing factor MCM7
+Macromolecule #7: DNA replication complex GINS protein PSF1
+Macromolecule #8: DNA replication complex GINS protein PSF2
+Macromolecule #9: DNA replication complex GINS protein PSF3
+Macromolecule #10: DNA replication complex GINS protein SLD5
+Macromolecule #11: Cell division control protein 45
+Macromolecule #12: DNA polymerase alpha-binding protein
+Macromolecule #15: Topoisomerase 1-associated factor 1
+Macromolecule #16: Chromosome segregation in meiosis protein 3
+Macromolecule #13: DNA fork, leading-strand template
+Macromolecule #14: DNA fork, lagging-strand template
+Macromolecule #17: ZINC ION
+Macromolecule #18: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
+Macromolecule #19: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 5 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 5 sec. / Pretreatment - Atmosphere: AIR / Details: 15 mA | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 277.15 K / Instrument: HOMEMADE PLUNGER Details: Three microlitres of sample was applied on a grid and incubated for 15-30 s at 4 degC before manually blotting with filter paper for 10 s and plunge-freezing in liquid ethane.. | |||||||||||||||||||||
| Details | In vitro reconstitution from individual components: CMG, Csm3-Tof1, Mrc1, Ctf4 and a DNA fork |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-20 / Number grids imaged: 2 / Number real images: 6682 / Average exposure time: 7.0 sec. / Average electron dose: 37.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 0.5 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.4000000000000001 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 20 / Target criteria: FSC 0.5 | ||||||||
| Output model |  PDB-6skl: |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)