+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-0833 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | cryo-em structure of alpha-synuclein fiber mutation type E46K | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | alpha-syn fiber / Parkinson disease / PROTEIN FIBRIL | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber ...negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / regulation of synaptic vesicle recycling / mitochondrial membrane organization / negative regulation of chaperone-mediated autophagy / regulation of reactive oxygen species biosynthetic process / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / positive regulation of neurotransmitter secretion / SNARE complex assembly / regulation of norepinephrine uptake / regulation of locomotion / negative regulation of dopamine metabolic process / positive regulation of inositol phosphate biosynthetic process / regulation of macrophage activation / transporter regulator activity / negative regulation of microtubule polymerization / synaptic vesicle transport / synaptic vesicle priming / dopamine uptake involved in synaptic transmission / protein kinase inhibitor activity / mitochondrial ATP synthesis coupled electron transport / regulation of dopamine secretion / dynein complex binding / positive regulation of receptor recycling / negative regulation of thrombin-activated receptor signaling pathway / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of endocytosis / positive regulation of exocytosis / synaptic vesicle exocytosis / kinesin binding / synaptic vesicle endocytosis / enzyme inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of serotonin uptake / response to type II interferon / regulation of presynapse assembly / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / supramolecular fiber organization / phospholipid metabolic process / cellular response to fibroblast growth factor stimulus / inclusion body / axon terminus / Hsp70 protein binding / cellular response to epinephrine stimulus / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / adult locomotory behavior / SNARE binding / excitatory postsynaptic potential / protein tetramerization / phosphoprotein binding / fatty acid metabolic process / microglial cell activation / ferrous iron binding / regulation of long-term neuronal synaptic plasticity / synapse organization / protein destabilization / PKR-mediated signaling / phospholipid binding / receptor internalization / tau protein binding / long-term synaptic potentiation / terminal bouton / positive regulation of inflammatory response / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress / neuron apoptotic process / cell cortex / histone binding / response to lipopolysaccharide / microtubule binding / molecular adaptor activity / chemical synaptic transmission / amyloid fibril formation / mitochondrial outer membrane / negative regulation of neuron apoptotic process / oxidoreductase activity 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
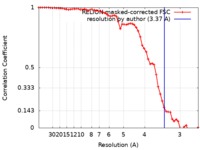
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 3.37 Å | |||||||||
 データ登録者 データ登録者 | Li YW / Zhao K | |||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2020 ジャーナル: Nat Commun / 年: 2020タイトル: Parkinson's disease associated mutation E46K of α-synuclein triggers the formation of a distinct fibril structure. 著者: Kun Zhao / Yaowang Li / Zhenying Liu / Houfang Long / Chunyu Zhao / Feng Luo / Yunpeng Sun / Youqi Tao / Xiao-Dong Su / Dan Li / Xueming Li / Cong Liu /  要旨: Amyloid aggregation of α-synuclein (α-syn) is closely associated with Parkinson's disease (PD) and other synucleinopathies. Several single amino-acid mutations (e.g. E46K) of α-syn have been ...Amyloid aggregation of α-synuclein (α-syn) is closely associated with Parkinson's disease (PD) and other synucleinopathies. Several single amino-acid mutations (e.g. E46K) of α-syn have been identified causative to the early onset of familial PD. Here, we report the cryo-EM structure of an α-syn fibril formed by N-terminally acetylated E46K mutant α-syn (Ac-E46K). The fibril structure represents a distinct fold of α-syn, which demonstrates that the E46K mutation breaks the electrostatic interactions in the wild type (WT) α-syn fibril and thus triggers the rearrangement of the overall structure. Furthermore, we show that the Ac-E46K fibril is less resistant to harsh conditions and protease cleavage, and more prone to be fragmented with an enhanced seeding capability than that of the WT fibril. Our work provides a structural view to the severe pathology of the PD familial mutation E46K of α-syn and highlights the importance of electrostatic interactions in defining the fibril polymorphs. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_0833.map.gz emd_0833.map.gz | 5.8 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-0833-v30.xml emd-0833-v30.xml emd-0833.xml emd-0833.xml | 9.7 KB 9.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_0833_fsc.xml emd_0833_fsc.xml | 5.8 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_0833.png emd_0833.png | 135.3 KB | ||
| Filedesc metadata |  emd-0833.cif.gz emd-0833.cif.gz | 4.8 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0833 http://ftp.pdbj.org/pub/emdb/structures/EMD-0833 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0833 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0833 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_0833_validation.pdf.gz emd_0833_validation.pdf.gz | 514.3 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_0833_full_validation.pdf.gz emd_0833_full_validation.pdf.gz | 513.9 KB | 表示 | |
| XML形式データ |  emd_0833_validation.xml.gz emd_0833_validation.xml.gz | 8.9 KB | 表示 | |
| CIF形式データ |  emd_0833_validation.cif.gz emd_0833_validation.cif.gz | 11.1 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0833 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0833 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0833 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0833 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_0833.map.gz / 形式: CCP4 / 大きさ: 15.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_0833.map.gz / 形式: CCP4 / 大きさ: 15.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.33 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : alpha-synuclein fiber mutation type E46K
| 全体 | 名称: alpha-synuclein fiber mutation type E46K |
|---|---|
| 要素 |
|
-超分子 #1: alpha-synuclein fiber mutation type E46K
| 超分子 | 名称: alpha-synuclein fiber mutation type E46K / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Alpha-synuclein
| 分子 | 名称: Alpha-synuclein / タイプ: protein_or_peptide / ID: 1 / コピー数: 6 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 5.402157 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: KKGVVHGVAT VAEKTKEQVT NVGGAVVTGV TAVAQKTVEG AGSIAAATGF VKKDQ UniProtKB: Alpha-synuclein |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 289 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: SUPER-RESOLUTION / デジタル化 - 画像ごとのフレーム数: 1-32 / 平均露光時間: 8.0 sec. / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)