[English] 日本語
 Yorodumi
Yorodumi- EMDB-0826: Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0826 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium | |||||||||
 Map data Map data | postprocess_masked map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Gap junction / Hemichannel / Hexamer / ATP release / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationconnexin complex / gap junction channel activity / myelination / sensory perception of sound / cell-cell signaling / myelin sheath / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
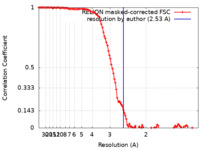
| Method | single particle reconstruction / cryo EM / Resolution: 2.53 Å | |||||||||
 Authors Authors | Lee HJ / Jeong H | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2020 Journal: Sci Adv / Year: 2020Title: Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Authors: Hyuk-Joon Lee / Hyeongseop Jeong / Jaekyung Hyun / Bumhan Ryu / Kunwoong Park / Hyun-Ho Lim / Jejoong Yoo / Jae-Sung Woo /   Abstract: Connexin family proteins assemble into hexameric channels called hemichannels/connexons, which function as transmembrane channels or dock together to form gap junction intercellular channels (GJIChs). ...Connexin family proteins assemble into hexameric channels called hemichannels/connexons, which function as transmembrane channels or dock together to form gap junction intercellular channels (GJIChs). We determined the cryo-electron microscopy structures of human connexin 31.3 (Cx31.3)/GJC3 hemichannels in the presence and absence of calcium ions and with a hearing-loss mutation R15G at 2.3-, 2.5-, and 2.6-Å resolutions, respectively. Compared with available structures of GJICh in open conformation, Cx31.3 hemichannel shows substantial structural changes of highly conserved regions in the connexin family, including opening of calcium ion-binding tunnels, reorganization of salt-bridge networks, exposure of lipid-binding sites, and collocation of amino-terminal helices at the cytoplasmic entrance. We also found that the hemichannel has a pore with a diameter of ~8 Å and selectively transports chloride ions. Our study provides structural insights into the permeant selectivity of Cx31.3 hemichannel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0826.map.gz emd_0826.map.gz | 13.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0826-v30.xml emd-0826-v30.xml emd-0826.xml emd-0826.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0826_fsc.xml emd_0826_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_0826.png emd_0826.png | 157.9 KB | ||
| Filedesc metadata |  emd-0826.cif.gz emd-0826.cif.gz | 5.4 KB | ||
| Others |  emd_0826_half_map_1.map.gz emd_0826_half_map_1.map.gz emd_0826_half_map_2.map.gz emd_0826_half_map_2.map.gz | 96.9 MB 96.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0826 http://ftp.pdbj.org/pub/emdb/structures/EMD-0826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0826 | HTTPS FTP |
-Related structure data
| Related structure data |  6l3uMC  0825C  0827C  6l3tC  6l3vC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0826.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0826.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocess_masked map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.673 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half map 2
| File | emd_0826_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 1
| File | emd_0826_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium
| Entire | Name: Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium |
|---|---|
| Components |
|
-Supramolecule #1: Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium
| Supramolecule | Name: Human Cx31.3/GJC3 connexin hemichannel in the presence of calcium type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Gap junction gamma-3 protein
| Macromolecule | Name: Gap junction gamma-3 protein / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 31.339365 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MCGRFLRRLL AEESRRSTPV GRLLLPVLLG FRLVLLAASG PGVYGDEQSE FVCHTQQPGC KAACFDAFHP LSPLRFWVFQ VILVAVPSA LYMGFTLYHV IWHWELSGKG KEEETLIQGR EGNTDVPGAG SLRLLWAYVA QLGARLVLEG AALGLQYHLY G FQMPSSFA ...String: MCGRFLRRLL AEESRRSTPV GRLLLPVLLG FRLVLLAASG PGVYGDEQSE FVCHTQQPGC KAACFDAFHP LSPLRFWVFQ VILVAVPSA LYMGFTLYHV IWHWELSGKG KEEETLIQGR EGNTDVPGAG SLRLLWAYVA QLGARLVLEG AALGLQYHLY G FQMPSSFA CRREPCLGSI TCNLSRPSEK TIFLKTMFGV SGFCLLFTFL ELVLLGLGRW WRTWKHKSSS SKYFLTSEST RR HKKATDS LPVVETKEQF QEAVPGRSLA QEKQRPVGPR DA UniProtKB: Gap junction gamma-3 protein |
-Macromolecule #2: Lauryl Maltose Neopentyl Glycol
| Macromolecule | Name: Lauryl Maltose Neopentyl Glycol / type: ligand / ID: 2 / Number of copies: 6 / Formula: LMN |
|---|---|
| Molecular weight | Theoretical: 1.005188 KDa |
| Chemical component information |  ChemComp-AV0: |
-Macromolecule #3: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 3 / Number of copies: 6 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 174 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)