+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0415 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | T.elongatus NDH (data-set 1) | ||||||||||||
 Map data Map data | Calibrated voxel size of 1.068 | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | photosynthesis / bioenergetics / membrane protein complex / OXIDOREDUCTASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationTranslocases; Catalysing the translocation of protons; Linked to oxidoreductase reactions / NADH dehydrogenase complex / transmembrane transporter complex / photosynthetic electron transport chain / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / plasma membrane-derived thylakoid membrane / photosynthesis, light reaction / ubiquinone binding / electron transport coupled proton transport / NADH dehydrogenase activity ...Translocases; Catalysing the translocation of protons; Linked to oxidoreductase reactions / NADH dehydrogenase complex / transmembrane transporter complex / photosynthetic electron transport chain / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / plasma membrane-derived thylakoid membrane / photosynthesis, light reaction / ubiquinone binding / electron transport coupled proton transport / NADH dehydrogenase activity / respiratory chain complex I / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / endomembrane system / aerobic respiration / NAD binding / 4 iron, 4 sulfur cluster binding / iron ion binding / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Thermosynechococcus elongatus BP-1 (bacteria) / Thermosynechococcus elongatus BP-1 (bacteria) /   Thermosynechococcus elongatus (strain BP-1) (bacteria) Thermosynechococcus elongatus (strain BP-1) (bacteria) | ||||||||||||
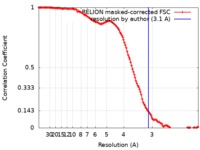
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Laughlin TG / Bayne A | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Structure of the complex I-like molecule NDH of oxygenic photosynthesis. Authors: Thomas G Laughlin / Andrew N Bayne / Jean-François Trempe / David F Savage / Karen M Davies /   Abstract: Cyclic electron flow around photosystem I (PSI) is a mechanism by which photosynthetic organisms balance the levels of ATP and NADPH necessary for efficient photosynthesis. NAD(P)H dehydrogenase-like ...Cyclic electron flow around photosystem I (PSI) is a mechanism by which photosynthetic organisms balance the levels of ATP and NADPH necessary for efficient photosynthesis. NAD(P)H dehydrogenase-like complex (NDH) is a key component of this pathway in most oxygenic photosynthetic organisms and is the last large photosynthetic membrane-protein complex for which the structure remains unknown. Related to the respiratory NADH dehydrogenase complex (complex I), NDH transfers electrons originating from PSI to the plastoquinone pool while pumping protons across the thylakoid membrane, thereby increasing the amount of ATP produced per NADP molecule reduced. NDH possesses 11 of the 14 core complex I subunits, as well as several oxygenic-photosynthesis-specific (OPS) subunits that are conserved from cyanobacteria to plants. However, the three core complex I subunits that are involved in accepting electrons from NAD(P)H are notably absent in NDH, and it is therefore not clear how NDH acquires and transfers electrons to plastoquinone. It is proposed that the OPS subunits-specifically NdhS-enable NDH to accept electrons from its electron donor, ferredoxin. Here we report a 3.1 Å structure of the 0.42-MDa NDH complex from the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1, obtained by single-particle cryo-electron microscopy. Our maps reveal the structure and arrangement of the principal OPS subunits in the NDH complex, as well as an unexpected cofactor close to the plastoquinone-binding site in the peripheral arm. The location of the OPS subunits supports a role in electron transfer and defines two potential ferredoxin-binding sites at the apex of the peripheral arm. These results suggest that NDH could possess several electron transfer routes, which would serve to maximize plastoquinone reduction and avoid deleterious off-target chemistry of the semi-plastoquinone radical. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0415.map.gz emd_0415.map.gz | 167 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0415-v30.xml emd-0415-v30.xml emd-0415.xml emd-0415.xml | 38.4 KB 38.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0415_fsc.xml emd_0415_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_0415.png emd_0415.png | 127.5 KB | ||
| Masks |  emd_0415_msk_1.map emd_0415_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-0415.cif.gz emd-0415.cif.gz | 9.9 KB | ||
| Others |  emd_0415_half_map_1.map.gz emd_0415_half_map_1.map.gz emd_0415_half_map_2.map.gz emd_0415_half_map_2.map.gz | 140.7 MB 140.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0415 http://ftp.pdbj.org/pub/emdb/structures/EMD-0415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0415 | HTTPS FTP |
-Related structure data
| Related structure data |  6nbqMC  6nbyMC  0416C  0417C  0418C  0419C  0420C  0425C  6nbxC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10257 (Title: Structure of NDH the complex I-like molecule of photosynthesis EMPIAR-10257 (Title: Structure of NDH the complex I-like molecule of photosynthesisData size: 1.8 TB Data #1: Unaligned multiframe micrographs for NDH dataset1 [micrographs - multiframe] Data #2: Unaligned multiframe micrographs for NDH dataset2 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0415.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0415.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Calibrated voxel size of 1.068 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.068 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
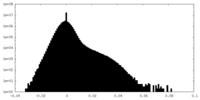
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_0415_msk_1.map emd_0415_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
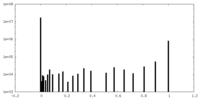
| Density Histograms |
-Half map: em-half-volume P1
| File | emd_0415_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | em-half-volume_P1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
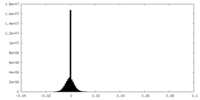
| Density Histograms |
-Half map: em-half-volume P2
| File | emd_0415_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | em-half-volume_P2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
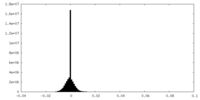
| Density Histograms |
- Sample components
Sample components
+Entire : NAD(P)H dehydrogenase-like complex (NDH/NDH-1_1/NDH1L) from T.elo...
+Supramolecule #1: NAD(P)H dehydrogenase-like complex (NDH/NDH-1_1/NDH1L) from T.elo...
+Macromolecule #1: NAD(P)H-quinone oxidoreductase subunit H
+Macromolecule #2: NAD(P)H-quinone oxidoreductase subunit J
+Macromolecule #3: NAD(P)H-quinone oxidoreductase subunit K
+Macromolecule #4: NAD(P)H-quinone oxidoreductase subunit I
+Macromolecule #5: NAD(P)H-quinone oxidoreductase subunit N
+Macromolecule #6: NAD(P)H-quinone oxidoreductase subunit L
+Macromolecule #7: NAD(P)H-quinone oxidoreductase subunit M
+Macromolecule #8: NAD(P)H-quinone oxidoreductase subunit O
+Macromolecule #9: Tlr0636 protein
+Macromolecule #10: NdhA
+Macromolecule #11: NAD(P)H-quinone oxidoreductase subunit 3
+Macromolecule #12: NAD(P)H-quinone oxidoreductase subunit 4L
+Macromolecule #13: Proton-translocating NADH-quinone dehydrogenase subunit P NdhP
+Macromolecule #14: NADH dehydrogenase subunit 5
+Macromolecule #15: NAD(P)H-quinone oxidoreductase chain 4 1
+Macromolecule #16: NAD(P)H-quinone oxidoreductase subunit 2
+Macromolecule #17: NADH-quinone oxidoreductase subunit J
+Macromolecule #18: IRON/SULFUR CLUSTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6 Component:
| ||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 7 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: OTHER | ||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV Details: incubate on grid for 30 seconds and blot 2.5 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-35 / Number grids imaged: 1 / Number real images: 2519 / Average exposure time: 0.2 sec. / Average electron dose: 50.0 e/Å2 / Details: One image per hole, focusing at each image. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | Homology models were generated from appropriate chain in 4HEA and 3C4S and rigid-body docked into the map using PHENIX. Remaining chains were built ab initio in COOT. All chains were iteratively refined and adjusted using PHENIX and COOT, respectively. | ||||||
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 30 / Target criteria: Corelation coefficient | ||||||
| Output model |  PDB-6nbq:  PDB-6nby: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)