+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The baseplate complex from the type VI secretion system | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Secretion / baseplate / complex / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationType VI secretion system protein TssE1-like / Type VI secretion system TssF / Type VI secretion system, TssF / Type VI secretion, TssG-like / Type VI secretion, TssG / IraD/Gp25-like / Baseplate wedge protein gp25 Similarity search - Domain/homology | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.7 Å | |||||||||
 Authors Authors | Rapisarda C / Fronzes R | |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2018 Journal: Nat Microbiol / Year: 2018Title: Biogenesis and structure of a type VI secretion baseplate. Authors: Yassine Cherrak / Chiara Rapisarda / Riccardo Pellarin / Guillaume Bouvier / Benjamin Bardiaux / Fabrice Allain / Christian Malosse / Martial Rey / Julia Chamot-Rooke / Eric Cascales / Rémi ...Authors: Yassine Cherrak / Chiara Rapisarda / Riccardo Pellarin / Guillaume Bouvier / Benjamin Bardiaux / Fabrice Allain / Christian Malosse / Martial Rey / Julia Chamot-Rooke / Eric Cascales / Rémi Fronzes / Eric Durand /  Abstract: To support their growth in a competitive environment and cause pathogenesis, bacteria have evolved a broad repertoire of macromolecular machineries to deliver specific effectors and toxins. Among ...To support their growth in a competitive environment and cause pathogenesis, bacteria have evolved a broad repertoire of macromolecular machineries to deliver specific effectors and toxins. Among these multiprotein complexes, the type VI secretion system (T6SS) is a contractile nanomachine that targets both prokaryotic and eukaryotic cells. The T6SS comprises two functional subcomplexes: a bacteriophage-related tail structure anchored to the cell envelope by a membrane complex. As in other contractile injection systems, the tail is composed of an inner tube wrapped by a sheath and built on the baseplate. In the T6SS, the baseplate is not only the tail assembly platform, but also docks the tail to the membrane complex and hence serves as an evolutionary adaptor. Here we define the biogenesis pathway and report the cryo-electron microscopy (cryo-EM) structure of the wedge protein complex of the T6SS from enteroaggregative Escherichia coli (EAEC). Using an integrative approach, we unveil the molecular architecture of the whole T6SS baseplate and its interaction with the tail sheath, offering detailed insights into its biogenesis and function. We discuss architectural and mechanistic similarities but also reveal key differences with the T4 phage and Mu phage baseplates. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0009.map.gz emd_0009.map.gz | 28.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0009-v30.xml emd-0009-v30.xml emd-0009.xml emd-0009.xml | 12.2 KB 12.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0009.png emd_0009.png | 55.2 KB | ||
| Filedesc metadata |  emd-0009.cif.gz emd-0009.cif.gz | 5.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0009 http://ftp.pdbj.org/pub/emdb/structures/EMD-0009 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0009 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0009 | HTTPS FTP |
-Validation report
| Summary document |  emd_0009_validation.pdf.gz emd_0009_validation.pdf.gz | 259.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0009_full_validation.pdf.gz emd_0009_full_validation.pdf.gz | 258.5 KB | Display | |
| Data in XML |  emd_0009_validation.xml.gz emd_0009_validation.xml.gz | 5.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0009 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0009 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0009 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0009 | HTTPS FTP |
-Related structure data
| Related structure data |  6gj1MC  0008C  0010C  6giyC  6gj3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0009.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0009.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
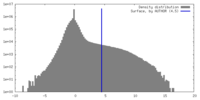
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Type VI secretion system baseplate complex
| Entire | Name: Type VI secretion system baseplate complex |
|---|---|
| Components |
|
-Supramolecule #1: Type VI secretion system baseplate complex
| Supramolecule | Name: Type VI secretion system baseplate complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Putative type VI secretion protein
| Macromolecule | Name: Putative type VI secretion protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 66.557125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDDLTLRYFD AEMRYLREAG KAFAQAHPDR AAMLDLDKAG TPDPCVERLF EGFAFSMGRL RQKIDDDLPE LTESLVSMLW PHYLRTIPS LSVVALTPRL SVMKMAETVP AGLEVTSRPV GPGNTVCRYR TTRAIPLNPL AVEKVVMTTE PDGRSVLKIG F ACSELADW ...String: MDDLTLRYFD AEMRYLREAG KAFAQAHPDR AAMLDLDKAG TPDPCVERLF EGFAFSMGRL RQKIDDDLPE LTESLVSMLW PHYLRTIPS LSVVALTPRL SVMKMAETVP AGLEVTSRPV GPGNTVCRYR TTRAIPLNPL AVEKVVMTTE PDGRSVLKIG F ACSELADW SQVDLHRLSL YLAAEAPVSS TLHLMMTKRL AALYLRLPGN DERIRIDGWF SPGGFAEEDR LWPKGDSAFS GY QLLLEYF TFREKFMFVH LNGLENVSLP AGISGFDLEV VLSQPWPADL PVTDDALCLH CVPVINLFTL EADPLIINGL ESE YLLRPK RLQDGYTEIY SVDAVTGSGR TGSAEYVPFT SFRHRGGMLR HDAPERYYHT RVKRGVTGMY DTWLILGGQR WEAD RMPER ETLSLRITGT NGQLPRRALQ STLLDRCEQV LQAPVSVRNL CKPTLPVYPP TEDRFHWRVM SHLGTGFLNM LSSAE VLRG TLALYNWRDD ELNHRRLDAI LAVQHHRIQR FEKGFLLRGL DVEVTLDGNG FAGEGDIHLF GEMLNRFLAL YADMNQ FNQ LTLIVQPEGK CIRWKENHNP RLPG UniProtKB: Type VI secretion protein |
-Macromolecule #2: TssG
| Macromolecule | Name: TssG / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.216344 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHPVERKSQS APARLITRYR KQLPYINFYR FCQLLEQSQP DQPPIGSGWQ ARQEAVRFCP YPGMGFPASE IKDAVIPEES HLPPIVHVT FMGLYGVTSP LPAHYISDIA QQREGHEAAA DFLDIFSHRL ITQYYRIWRK YSYPATFEAG GQDKTSQYLL G LARLGIPG ...String: MHPVERKSQS APARLITRYR KQLPYINFYR FCQLLEQSQP DQPPIGSGWQ ARQEAVRFCP YPGMGFPASE IKDAVIPEES HLPPIVHVT FMGLYGVTSP LPAHYISDIA QQREGHEAAA DFLDIFSHRL ITQYYRIWRK YSYPATFEAG GQDKTSQYLL G LARLGIPG CAQNIATPVS RFLALLPLML LPGRTAEGLT SLVTLLAPGT QARVWHHDRR RIPLKTPLTM RVHHPVSLKS RP VMGDHAT DVNGQVLLQL STQTGSEVQG WLPGGHLYSD LLALLHVYLG SRLDVRLQLC VERSLLPDAR LSCRPAAGSP QLG RTAVMR TQAKIATSAA RVMTISLGRY QRVQEHYQRK ETQENGDYRW UniProtKB: Type VI secretion system baseplate subunit TssG |
-Macromolecule #3: TssE
| Macromolecule | Name: TssE / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 16.320783 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPRPSLYEIL YGNFTGGLEL NQVGEEEQVI LSVLDNMQRI LNTRAGSLKH LPDYGLPDIT TILQGMPGTA HQLMRVLSDV LLKYEPRIK RVDVTMQEQT QPGELHYVID AELKDAGLVR YGTTFIPEGR VLLRHLKQQR YVQT UniProtKB: IraD/Gp25-like domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 4.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 32504 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT / Software - Name: cryoSPARC |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: RELION |
 Movie
Movie Controller
Controller












 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)