+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: SASBDB / ID: SASDGY4 |
|---|---|
 試料 試料 | Beta-amylase 2, chloroplastic (AtBAM2)
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報beta-amylase / beta-amylase activity / chloroplast stroma / polysaccharide catabolic process / chloroplast 類似検索 - 分子機能 |
| 生物種 |  |
 引用 引用 |  日付: 2019 Aug 30 日付: 2019 Aug 30タイトル: Solution structure and assembly of β-amylase 2 from Arabidopsis thaliana 著者: Chandrasekharan N / Ravenburg C / Roy I / Monroe J |
 登録者 登録者 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-モデル
| モデル #3698 |  タイプ: atomic / カイ2乗値: 8.96285214274195  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
|---|---|
| モデル #3699 |  タイプ: dummy / 対称性: P222 / カイ2乗値: 1.311  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
| モデル #3700 |  タイプ: dummy / ソフトウェア: (dammif (r10552)) / カイ2乗値: 2.189  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
- 試料
試料
 試料 試料 | 名称: Beta-amylase 2, chloroplastic (AtBAM2) / 試料濃度: 1.65-2.75 |
|---|---|
| バッファ | 名称: 50 mM HEPES / pH: 7.5 |
| 要素 #1874 | 名称: BAM2 / タイプ: protein / 記述: Beta-amylase 2, chloroplastic / 分子量: 57.141 / 分子数: 4 / 由来: Arabidopsis thaliana / 参照: UniProt: O65258 配列: MGSSHHHHHH SQDPAESTEE DRVPIDDDDD STDQLVDEEI VHFEERDFAG TACVPVYVML PLGVIDMNSE VVEPEELLDQ LRTLKSVNVD GVMVDCWWGI VESHTPQVYN WSGYKKLFQM IRELGLKIQV VMSFHECGGN VGDDVHIQIP EWVREIGQSN PDIYFTDSAG ...配列: MGSSHHHHHH SQDPAESTEE DRVPIDDDDD STDQLVDEEI VHFEERDFAG TACVPVYVML PLGVIDMNSE VVEPEELLDQ LRTLKSVNVD GVMVDCWWGI VESHTPQVYN WSGYKKLFQM IRELGLKIQV VMSFHECGGN VGDDVHIQIP EWVREIGQSN PDIYFTDSAG RRNTECLTWG IDKQRVLRGR TALEVYFDYM RSFRVEFDEF FEEKIIPEIE VGLGPCGELR YPSYPAQFGW KYPGIGEFQC YDKYLMNSLK EAAEVRGHSF WGRGPDNTET YNSTPHGTGF FRDGGDYDSY YGRFFLNWYS RVLIDHGDRV LAMANLAFEG TCIAAKLSGI HWWYKTASHA AELTAGFYNS SNRDGYGPIA AMFKKHDAAL NFTCVELRTL DQHEDFPEAL ADPEGLVWQV LNAAWDASIP VASENALPCY DREGYNKILE NAKPLTDPDG RHLSCFTYLR LNPTLMESQN FKEFERFLKR MHGEAVPDLG LAPGTQETNP E |
-実験情報
| ビーム | 設備名称: Advanced Light Source (ALS) 12.3.1 (SIBYLS) / 地域: Berkeley, CA / 国: USA  / 線源: X-ray synchrotron / 波長: 0.127 Å / スペクトロメータ・検出器間距離: 2 mm / 線源: X-ray synchrotron / 波長: 0.127 Å / スペクトロメータ・検出器間距離: 2 mm | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: Pilatus3 X 2M / Pixsize x: 172 mm | |||||||||||||||||||||||||||||||||
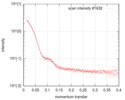
| スキャン |
| |||||||||||||||||||||||||||||||||
| 距離分布関数 P(R) |
| |||||||||||||||||||||||||||||||||
| 結果 | コメント: We expressed Arabidopsis thaliana AtBAM2 in E. coli BL21 cells from a pETDuet-1 expression vector with an N-terminal 6-His tag as constructed by (Monroe et al., 2017, 2018). We induced ...コメント: We expressed Arabidopsis thaliana AtBAM2 in E. coli BL21 cells from a pETDuet-1 expression vector with an N-terminal 6-His tag as constructed by (Monroe et al., 2017, 2018). We induced AtBAM2 expression in BL21 E coli at OD600 with 0.3 mM IPTG in 2xYT broth with 60 µg/ml ampicillin at 30 oC overnight. Cells were lysed and sonicated in a buffer containing 50 mM NaH2PO4, pH 8, 500 mM NaCl, and 2 mM imidazole. The supernatant was loaded onto a TALON cobalt column using an AKTA Start and washed with a buffer containing 50 mM HEPES pH 8, 500 mM NaCl, 5% glycerol, 10 mM TCEP, and 40 mM imidazole. The protein was then eluted with buffer containing 50 mM HEPES pH 8, 500 mM NaCl, 5% glycerol, 10 mM TCEP, and 500 mM imidazole. Pure protein, as determined by a band present at ∼50 kDa on a 4–20% Tris‐Glycine gel stained with Coomassie Blue, was concentrated in a Spin‐X UF concentrator with a 5000 MWCO. The concentrated protein was then further purified using a HiLoad 16/60 column filled with Superdex 200 in 50 mM HEPES, pH 7. Pure protein based on SDS-PAGE was concentrated as before and the concentration of protein was determined via absorbance at 280 nm using an extinction coefficient of 94310 M−1 cm−1 which was calculated from the sequence using ProtParam (Gasteiger et al., 2005) Matching buffer exposures were collected before and after samples to ensure there was no difference in the scattering due to contamination of the sample cell. Scattering data were subtracted from buffer and then processed in PRIMUS (ATSAS 2.8.4 r10553) to create an average data file (Konarev et al., 2003). Data were analyzed using PRIMUS, GNOM, and SCÅTTER (v3.0g) to determine dimensions and create a merged data file from the two data sets at 30 and 50 µM AtBAM2 (Konarev et al., 2003). We then used the merged data file in DAMMIF (v1.1.2) and DAMMIN (v5.3) to generate the dummy-atom model and aligned the result to the all-atom structure using SASTBX (Svergun, 1992, 1999; Svergun et al., 2001; Franke & Svergun, 2009; Liu et al., 2012). Dummy atom models from different methods were aligned and visualized using YASARA Structure (Krieger et al., 2009).
|
 ムービー
ムービー コントローラー
コントローラー


 SASDGY4
SASDGY4