+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  |
|---|---|
 試料 試料 | AMP/fumarate-bound human adenylosuccinate lyase (ADSL)
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報AMP biosynthetic process / adenylosuccinate lyase / N6-(1,2-dicarboxyethyl)AMP AMP-lyase (fumarate-forming) activity / (S)-2-(5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido) succinate lyase (fumarate-forming) activity / 'de novo' XMP biosynthetic process / Purine ribonucleoside monophosphate biosynthesis / 'de novo' AMP biosynthetic process / AMP salvage / purine nucleotide biosynthetic process / GMP biosynthetic process ...AMP biosynthetic process / adenylosuccinate lyase / N6-(1,2-dicarboxyethyl)AMP AMP-lyase (fumarate-forming) activity / (S)-2-(5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido) succinate lyase (fumarate-forming) activity / 'de novo' XMP biosynthetic process / Purine ribonucleoside monophosphate biosynthesis / 'de novo' AMP biosynthetic process / AMP salvage / purine nucleotide biosynthetic process / GMP biosynthetic process / 'de novo' IMP biosynthetic process / response to muscle activity / response to starvation / response to nutrient / aerobic respiration / response to hypoxia / protein-containing complex / identical protein binding / cytosol 類似検索 - 分子機能 |
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) |
 引用 引用 |  ジャーナル: Sci Rep / 年: 2018 ジャーナル: Sci Rep / 年: 2018タイトル: Molecular comparison of Neanderthal and Modern Human adenylosuccinate lyase. 著者: Bart Van Laer / Ulrike Kapp / Montserrat Soler-Lopez / Kaja Moczulska / Svante Pääbo / Gordon Leonard / Christoph Mueller-Dieckmann /    要旨: The availability of genomic data from extinct homini such as Neanderthals has caused a revolution in palaeontology allowing the identification of modern human-specific protein substitutions. ...The availability of genomic data from extinct homini such as Neanderthals has caused a revolution in palaeontology allowing the identification of modern human-specific protein substitutions. Currently, little is known as to how these substitutions alter the proteins on a molecular level. Here, we investigate adenylosuccinate lyase, a conserved enzyme involved in purine metabolism for which several substitutions in the modern human protein (hADSL) have been described to affect intelligence and behaviour. During evolution, modern humans acquired a specific substitution (Ala429Val) in ADSL distinguishing it from the ancestral variant present in Neanderthals (nADSL). We show here that despite this conservative substitution being solvent exposed and located distant from the active site, there is a difference in thermal stability, but not enzymology or ligand binding between nADSL and hADSL. Substitutions near residue 429 which do not profoundly affect enzymology were previously reported to cause neurological symptoms in humans. This study also reveals that ADSL undergoes conformational changes during catalysis which, together with the crystal structure of a hitherto undetermined product bound conformation, explains the molecular origin of disease for several modern human ADSL mutants. |
 登録者 登録者 |
|
- 構造の表示
構造の表示
- ダウンロードとリンク
ダウンロードとリンク
-Data source
| SASBDBのページ |  SASDEH5 SASDEH5 |
|---|
-関連構造データ
| 関連構造データ |  5nx8C  5nx9C  5nxaC C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- 外部リンク
外部リンク
| 「今月の分子」の関連する項目 |
|---|
-モデル
- 試料
試料
 試料 試料 | 名称: AMP/fumarate-bound human adenylosuccinate lyase (ADSL) 試料濃度: 5 mg/ml |
|---|---|
| バッファ | 名称: 10 mM HEPES, 100 mM NaCl, 1 mM DTT, 1mM AMP, 1mM Fumarate pH: 7.5 |
| 要素 #1310 | 名称: ADSL / タイプ: protein / 記述: Adenylosuccinate Lyase / 分子量: 55.17 / 分子数: 4 / 由来: Homo sapiens / 参照: UniProt: P30566 配列: GSHMAAGGDH GSPDSYRSPL ASRYASPEMC FVFSDRYKFR TWRQLWLWLA EAEQTLGLPI TDEQIQEMKS NLENIDFKMA AEEEKRLRHD VMAHVHTFGH CCPKAAGIIH LGATSCYVGD NTDLIILRNA LDLLLPKLAR VISRLADFAK ERASLPTLGF THFQPAQLTT ...配列: GSHMAAGGDH GSPDSYRSPL ASRYASPEMC FVFSDRYKFR TWRQLWLWLA EAEQTLGLPI TDEQIQEMKS NLENIDFKMA AEEEKRLRHD VMAHVHTFGH CCPKAAGIIH LGATSCYVGD NTDLIILRNA LDLLLPKLAR VISRLADFAK ERASLPTLGF THFQPAQLTT VGKRCCLWIQ DLCMDLQNLK RVRDDLRFRG VKGTTGTQAS FLQLFEGDDH KVEQLDKMVT EKAGFKRAFI ITGQTYTRKV DIEVLSVLAS LGASVHKICT DIRLLANLKE MEEPFEKQQI GSSAMPYKRN PMRSERCCSL ARHLMTLVMD PLQTASVQWF ERTLDDSANR RICLAEAFLT ADTILNTLQN ISEGLVVYPK VIERRIRQEL PFMATENIIM AMVKAGGSRQ DCHEKIRVLS QQAASVVKQE GGDNDLIERI QVDAYFSPIH SQLDHLLDPS SFTGRASQQV QRFLEEEVYP LLKPYESVMK VKAELCL |
-実験情報
| ビーム | 設備名称: ESRF BM29 / 地域: Grenoble / 国: France  / 線源: X-ray synchrotron / 波長: 0.099 Å / スペクトロメータ・検出器間距離: 2.864 mm / 線源: X-ray synchrotron / 波長: 0.099 Å / スペクトロメータ・検出器間距離: 2.864 mm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: Pilatus 1M / タイプ: Dectris / Pixsize x: 172 mm | |||||||||||||||
| スキャン | 測定日: 2016年7月24日 / 保管温度: 20 °C / セル温度: 20 °C / 照射時間: 1 sec. / フレーム数: 10 / 単位: 1/nm /
| |||||||||||||||
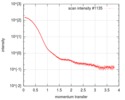
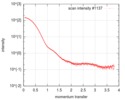
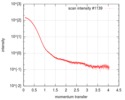
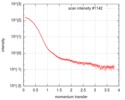
| 結果 | I0 from Guinier: 152 / Rg from Guinier: 3.62 nm / カーブのタイプ: single_conc /
|
 ムービー
ムービー コントローラー
コントローラー