+ Open data
Open data
- Basic information
Basic information
| Entry |  |
|---|---|
 Sample Sample | MsbA in stealth nanodisc (SANS, 100% D2O) + 1 mM ADP
|
| Function / homology |  Function and homology information Function and homology informationMsbA transporter complex / lipopolysaccharide floppase activity / lipid translocation / ABC-type lipid A-core oligosaccharide transporter / lipopolysaccharide transport / ATPase-coupled lipid transmembrane transporter activity / ABC-type xenobiotic transporter activity / lipid transport / ATP-binding cassette (ABC) transporter complex / transmembrane transport ...MsbA transporter complex / lipopolysaccharide floppase activity / lipid translocation / ABC-type lipid A-core oligosaccharide transporter / lipopolysaccharide transport / ATPase-coupled lipid transmembrane transporter activity / ABC-type xenobiotic transporter activity / lipid transport / ATP-binding cassette (ABC) transporter complex / transmembrane transport / lipid binding / ATP hydrolysis activity / ATP binding / identical protein binding / membrane / plasma membrane Similarity search - Function |
| Biological species |  Escherichia coli. (AL95 strain) |
 Citation Citation |  Journal: Structure / Year: 2018 Journal: Structure / Year: 2018Title: Conformational States of ABC Transporter MsbA in a Lipid Environment Investigated by Small-Angle Scattering Using Stealth Carrier Nanodiscs. Authors: Inokentijs Josts / Julius Nitsche / Selma Maric / Haydyn D Mertens / Martine Moulin / Michael Haertlein / Sylvain Prevost / Dmitri I Svergun / Sebastian Busch / V Trevor Forsyth / Henning Tidow /     Abstract: Structural studies of integral membrane proteins (IMPs) are challenging, as many of them are inactive or insoluble in the absence of a lipid environment. Here, we describe an approach making use of ...Structural studies of integral membrane proteins (IMPs) are challenging, as many of them are inactive or insoluble in the absence of a lipid environment. Here, we describe an approach making use of fractionally deuterium labeled "stealth carrier" nanodiscs that are effectively invisible to low-resolution neutron diffraction and enable structural studies of IMPs in a lipidic native-like solution environment. We illustrate the potential of the method in a joint small-angle neutron scattering (SANS) and X-ray scattering (SAXS) study of the ATP-binding cassette (ABC) transporter protein MsbA solubilized in the stealth nanodiscs. The data allow for a direct observation of the signal from the solubilized protein without contribution from the surrounding lipid nanodisc. Not only the overall shape but also differences between conformational states of MsbA can be reliably detected from the scattering data, demonstrating the sensitivity of the approach and its general applicability to structural studies of IMPs. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDDC5 SASDDC5 |
|---|
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- External links
External links
| Related items in Molecule of the Month |
|---|
-Models
- Sample
Sample
 Sample Sample | Name: MsbA in stealth nanodisc (SANS, 100% D2O) + 1 mM ADP / Contrast: -3.313 / Specific vol: 0.745 / Specimen concentration: 1.00-6.50 / Concentration method: A280 / Entity id: 1016 / 1017 / 1018 |
|---|---|
| Buffer | Name: 30 mM Tris, 150 mM NaCl, 1 mM ADP / pH: 7.5 / Comment: nanodisc buffer |
| Entity #1016 | Name: MsbA / Type: protein Description: Lipid A export ATP-binding/permease protein MsbA Formula weight: 66.279 / Num. of mol.: 2 / Source: Escherichia coli (strain K12) / References: UniProt: P60752 Sequence: MHHHHHHGEN LYFQGSHNDK DLSTWQTFRR LWPTIAPFKA GLIVAGVALI LNAASDTFML SLLKPLLDDG FGKTDRSVLV WMPLVVIGLM ILRGITSYVS SYCISWVSGK VVMTMRRRLF GHMMGMPVSF FDKQSTGTLL SRITYDSEQV ASSSSGALIT VVREGASIIG ...Sequence: MHHHHHHGEN LYFQGSHNDK DLSTWQTFRR LWPTIAPFKA GLIVAGVALI LNAASDTFML SLLKPLLDDG FGKTDRSVLV WMPLVVIGLM ILRGITSYVS SYCISWVSGK VVMTMRRRLF GHMMGMPVSF FDKQSTGTLL SRITYDSEQV ASSSSGALIT VVREGASIIG LFIMMFYYSW QLSIILIVLA PIVSIAIRVV SKRFRNISKN MQNTMGQVTT SAEQMLKGHK EVLIFGGQEV ETKRFDKVSN RMRLQGMKMV SASSISDPII QLIASLALAF VLYAASFPSV MDSLTAGTIT VVFSSMIALM RPLKSLTNVN AQFQRGMAAC QTLFTILDSE QEKDEGKRVI ERATGDVEFR NVTFTYPGRD VPALRNINLK IPAGKTVALV GRSGSGKSTI ASLITRFYDI DEGEILMDGH DLREYTLASL RNQVALVSQN VHLFNDTVAN NIAYARTEQY SREQIEEAAR MAYAMDFINK MDNGLDTVIG ENGVLLSGGQ RQRIAIARAL LRDSPILILD EATSALDTES ERAIQAALDE LQKNRTSLVI AHRLSTIEKA DEIVVVEDGV IVERGTHNDL LEHRGVYAQL HKMQFGQ |
| Entity #1017 | Name: d75-msp1d1 / Type: protein Description: Membrane scaffold protein 1D1 (deuterated, 75%) Formula weight: 24.661 / Num. of mol.: 2 Sequence: GHHHHHHHDY DIPTTENLYF QGSTFSKLRE QLGPVTQEFW DNLEKETEGL RQEMSKDLEE VKAKVQPYLD DFQKKWQEEM ELYRQKVEPL RAELQEGARQ KLHELQEKLS PLGEEMRDRA RAHVDALRTH LAPYSDELRQ RLAARLEALK ENGGARLAEY HAKATEHLST ...Sequence: GHHHHHHHDY DIPTTENLYF QGSTFSKLRE QLGPVTQEFW DNLEKETEGL RQEMSKDLEE VKAKVQPYLD DFQKKWQEEM ELYRQKVEPL RAELQEGARQ KLHELQEKLS PLGEEMRDRA RAHVDALRTH LAPYSDELRQ RLAARLEALK ENGGARLAEY HAKATEHLST LSEKAKPALE DLRQGLLPVL ESFKVSFLSA LEEYTKKLNT Q |
| Entity #1018 | Name: d(78,92)-POPC / Type: other Description: 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphocholine (deuteration: 78% head, 92% acyl) Formula weight: 0.76 / Source: Escherichia coli. (AL95 strain) Sequence: C42H82NO8P |
-Experimental information
| Beam | Instrument name: ILL D11 / City: Grenoble / 国: France  / Type of source: neutron source / Wavelength: 0.46 Å / Type of source: neutron source / Wavelength: 0.46 Å | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: CERCA / Type: 3-He gas detector / Pixsize x: 7.5 mm | ||||||||||||||||||||||||||||||||||||||||||
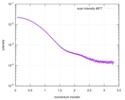
| Scan |
| ||||||||||||||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||||||||||||||||||||||||||
| Result | Comments: 'MsbA ABC transporter protein encapsulated in a partially deuterated
|
 Movie
Movie Controller
Controller