+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | 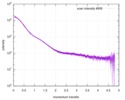 |
|---|---|
 試料 試料 | Small glutamine-rich tetratricopeptide repeat-containing protein alpha (full length; SGTA_FL)
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報TRC complex / negative regulation of ERAD pathway / tail-anchored membrane protein insertion into ER membrane / positive regulation of ERAD pathway / post-translational protein targeting to endoplasmic reticulum membrane / BAT3 complex binding / positive regulation of ubiquitin-dependent protein catabolic process / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of ubiquitin-dependent protein catabolic process / ERAD pathway ...TRC complex / negative regulation of ERAD pathway / tail-anchored membrane protein insertion into ER membrane / positive regulation of ERAD pathway / post-translational protein targeting to endoplasmic reticulum membrane / BAT3 complex binding / positive regulation of ubiquitin-dependent protein catabolic process / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of ubiquitin-dependent protein catabolic process / ERAD pathway / molecular adaptor activity / nucleoplasm / identical protein binding / nucleus / membrane / cytosol / cytoplasm 類似検索 - 分子機能 |
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) |
 引用 引用 |  ジャーナル: BMC Biol / 年: 2018 ジャーナル: BMC Biol / 年: 2018タイトル: Structural complexity of the co-chaperone SGTA: a conserved C-terminal region is implicated in dimerization and substrate quality control. 著者: Santiago Martínez-Lumbreras / Ewelina M Krysztofinska / Arjun Thapaliya / Alessandro Spilotros / Dijana Matak-Vinkovic / Enrico Salvadori / Peristera Roboti / Yvonne Nyathi / Janina H Muench ...著者: Santiago Martínez-Lumbreras / Ewelina M Krysztofinska / Arjun Thapaliya / Alessandro Spilotros / Dijana Matak-Vinkovic / Enrico Salvadori / Peristera Roboti / Yvonne Nyathi / Janina H Muench / Maxie M Roessler / Dmitri I Svergun / Stephen High / Rivka L Isaacson /   要旨: BACKGROUND: Protein quality control mechanisms are essential for cell health and involve delivery of proteins to specific cellular compartments for recycling or degradation. In particular, stray ...BACKGROUND: Protein quality control mechanisms are essential for cell health and involve delivery of proteins to specific cellular compartments for recycling or degradation. In particular, stray hydrophobic proteins are captured in the aqueous cytosol by a co-chaperone, the small glutamine-rich, tetratricopeptide repeat-containing protein alpha (SGTA), which facilitates the correct targeting of tail-anchored membrane proteins, as well as the sorting of membrane and secretory proteins that mislocalize to the cytosol and endoplasmic reticulum-associated degradation. Full-length SGTA has an unusual elongated dimeric structure that has, until now, evaded detailed structural analysis. The C-terminal region of SGTA plays a key role in binding a broad range of hydrophobic substrates, yet in contrast to the well-characterized N-terminal and TPR domains, there is a lack of structural information on the C-terminal domain. In this study, we present new insights into the conformation and organization of distinct domains of SGTA and show that the C-terminal domain possesses a conserved region essential for substrate processing in vivo. RESULTS: We show that the C-terminal domain region is characterized by α-helical propensity and an intrinsic ability to dimerize independently of the N-terminal domain. Based on the properties of ...RESULTS: We show that the C-terminal domain region is characterized by α-helical propensity and an intrinsic ability to dimerize independently of the N-terminal domain. Based on the properties of different regions of SGTA that are revealed using cell biology, NMR, SAXS, Native MS, and EPR, we observe that its C-terminal domain can dimerize in the full-length protein and propose that this reflects a closed conformation of the substrate-binding domain. CONCLUSION: Our results provide novel insights into the structural complexity of SGTA and provide a new basis for mechanistic studies of substrate binding and release at the C-terminal region. |
 登録者 登録者 |
|
- 構造の表示
構造の表示
- ダウンロードとリンク
ダウンロードとリンク
-Data source
| SASBDBのページ |  SASDDB6 SASDDB6 |
|---|
-関連構造データ
| 関連構造データ | C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- 外部リンク
外部リンク
| 「今月の分子」の関連する項目 |
|---|
-モデル
- 試料
試料
 試料 試料 | 名称: Small glutamine-rich tetratricopeptide repeat-containing protein alpha (full length; SGTA_FL) 試料濃度: 0.60-8.80 |
|---|---|
| バッファ | 名称: 10 mM potassium phosphate, 100 mM NaCl / pH: 6 |
| 要素 #1055 | 名称: SGTA_FL / タイプ: protein 記述: Small glutamine-rich tetratricopeptide repeat-containing protein alpha full length 分子量: 34.207 / 分子数: 2 / 由来: Homo sapiens / 参照: UniProt: O43765 配列: GSMDNKKRLA YAIIQFLHDQ LRHGGLSSDA QESLEVAIQC LETAFGVTVE DSDLALPQTL PEIFEAAATG KEMPQDLRSP ARTPPSEEDS AEAERLKTEG NEQMKVENFE AAVHFYGKAI ELNPANAVYF CNRAAAYSKL GNYAGAVQDC ERAICIDPAY SKAYGRMGLA ...配列: GSMDNKKRLA YAIIQFLHDQ LRHGGLSSDA QESLEVAIQC LETAFGVTVE DSDLALPQTL PEIFEAAATG KEMPQDLRSP ARTPPSEEDS AEAERLKTEG NEQMKVENFE AAVHFYGKAI ELNPANAVYF CNRAAAYSKL GNYAGAVQDC ERAICIDPAY SKAYGRMGLA LSSLNKHVEA VAYYKKALEL DPDNETYKSN LKIAELKLRE APSPTGGVGS FDIAGLLNNP GFMSMASNLM NNPQIQQLMS GMISGGNNPL GTPGTSPSQN DLASLIQAGQ QFAQQMQQQN PELIEQLRSQ IRSRTPSASN DDQQE |
-実験情報
| ビーム | 設備名称: PETRA III EMBL P12 / 地域: Hamburg / 国: Germany  / 線源: X-ray synchrotron / 波長: 0.124 Å / スペクトロメータ・検出器間距離: 3 mm / 線源: X-ray synchrotron / 波長: 0.124 Å / スペクトロメータ・検出器間距離: 3 mm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: Pilatus 2M | |||||||||||||||
| スキャン | 測定日: 2015年6月5日 / セル温度: 25 °C / 照射時間: 0.045 sec. / フレーム数: 20 / 単位: 1/nm /
| |||||||||||||||
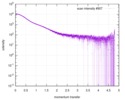
| 結果 | Experimental MW: 65 kDa / カーブのタイプ: merged
|
 ムービー
ムービー コントローラー
コントローラー