[English] 日本語
 Yorodumi
Yorodumi- SASDBW5: Cyclopentadecanone monooxygenase, NADP+ and cyclopentadecanone, w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  |
|---|---|
 Sample Sample | Cyclopentadecanone monooxygenase, NADP+ and cyclopentadecanone, wild-type
|
| Function / homology | Pyridine nucleotide-disulphide oxidoreductase / : / monooxygenase activity / FAD/NAD(P)-binding domain superfamily / Cyclopentadecanone 1,2-monooxygenase Function and homology information Function and homology information |
| Biological species |  Pseudomonas sp. HI-70 (bacteria) Pseudomonas sp. HI-70 (bacteria) |
 Citation Citation |  Journal: Biochim Biophys Acta / Year: 2016 Journal: Biochim Biophys Acta / Year: 2016Title: The role of conformational flexibility in Baeyer-Villiger monooxygenase catalysis and structure. Authors: Brahm J Yachnin / Peter C K Lau / Albert M Berghuis /  Abstract: BACKGROUND: The Baeyer-Villiger monooxygenases (BMVOs) are a group of microbial enzymes that have garnered interest as industrial biocatalysts. While great strides have been made in recent years to ...BACKGROUND: The Baeyer-Villiger monooxygenases (BMVOs) are a group of microbial enzymes that have garnered interest as industrial biocatalysts. While great strides have been made in recent years to understand the mechanism of these enzymes from a structural perspective, our understanding remains incomplete. In particular, the role of a twenty residue loop (residues 487-504), which we refer to as the "Control Loop," that is observed in either an ordered or disordered state in various crystal structures remains unclear. METHODS: Using SAXS, we have made the first observations of the Loop in solution with two BVMOs, cyclohexanone monooxygenase (CHMO) and cyclopentadecanone monooxygenase. We also made a series of ...METHODS: Using SAXS, we have made the first observations of the Loop in solution with two BVMOs, cyclohexanone monooxygenase (CHMO) and cyclopentadecanone monooxygenase. We also made a series of mutants of CHMO and analyzed them using SAXS, ITC, and an uncoupling assay. RESULTS: These experiments show that Control Loop ordering results in an overall more compact enzyme without altering global protein foldedness. We have also demonstrated that the Loop plays a ...RESULTS: These experiments show that Control Loop ordering results in an overall more compact enzyme without altering global protein foldedness. We have also demonstrated that the Loop plays a critical and complex role on enzyme structure and catalysis. The Control Loop appears to have a direct impact on the organization of the overall structure of the protein, as well as in influencing the active site environment. CONCLUSIONS: The data imply that the Loop can be divided into two regions, referred to as "sub-loops," that coordinate overall domain movements to changes in the active site. GENERAL SIGNIFICANCE: A better understanding of the mechanistic role of the Control Loop may ultimately be helpful in designing mutants with altered specificity and improved catalytic efficiency. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-Models
- Sample
Sample
 Sample Sample | Name: Cyclopentadecanone monooxygenase, NADP+ and cyclopentadecanone, wild-type Specimen concentration: 2.25-9.00 |
|---|---|
| Buffer | Name: 50mM Tris 2mM TCEP 5mM NADP+ 1mM cyclopentadecanon / pH: 8 |
| Entity #361 | Type: protein / Description: Cyclopentadecanone 1,2-monooxygenase / Formula weight: 69.13 / Num. of mol.: 1 / Source: Pseudomonas sp. HI-70 / References: UniProt: T2HVF7 Sequence: GSLEASMHMS QLIQEPAEAG VTSQKVSFDH VALREKYRQE RDKRLRQDGQ EQYLEVAVTC DEYLKDPYAD PIVRDPVVRE TDVFIIGGGF GGLLAAVRLQ QAGVSDYVMV ERAGDYGGTW YWNRYPGAQC DIESYVYMPL LEEMGYIPTE KYAFGTEILE YSRSIGRKFG ...Sequence: GSLEASMHMS QLIQEPAEAG VTSQKVSFDH VALREKYRQE RDKRLRQDGQ EQYLEVAVTC DEYLKDPYAD PIVRDPVVRE TDVFIIGGGF GGLLAAVRLQ QAGVSDYVMV ERAGDYGGTW YWNRYPGAQC DIESYVYMPL LEEMGYIPTE KYAFGTEILE YSRSIGRKFG LYERTYFQTE VKDLSWDDEA ARWRITTDRG DKFSARFVCM STGPLQRPKL PGIPGITSFK GHSFHTSRWD YSYTGGDQTG NLEGLKDKRV AIIGTGATSI QAVPHLAAYA QELYVIQRTP ISVGFRGNKP TDPEWAKSLQ PGWQQARMDN FNAITHGMPV DVDLVQDSWT KIFGEIGVFL GSDGSRAQMV DFQLMEQIRA RVDQEVKDPA TAESLKPYYN IMCKRPGFHD SYLPSFNKPN VTLVDTQGAG VERITEKGLV VNGREYEVDC LIYATGFEYQ TKLSRRNGYE IHGRNGQPLS DKWKDGLSTL WGYHIRDFPN CFILGNGQSA VTPNFTHMLN EAGKHVAYVV KHCLDERVDV FEPTAEAEQA WVDHVMSFAG IKQQYDRECT PSYYNNEGQV NDVALTRNNF YPGGAVAFIN ILREWREKGD FAQFQQRKR |
-Experimental information
| Beam | Instrument name: Advanced Light Source (ALS) 12.3.1 (SIBYLS) City: Berkeley, CA / 国: USA  / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 1.5 mm / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 1.5 mm | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: MAR 165 CCD | |||||||||||||||||||||
| Scan |
| |||||||||||||||||||||
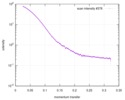
| Distance distribution function P(R) |
| |||||||||||||||||||||
| Result |
|
 Movie
Movie Controller
Controller


 SASDBW5
SASDBW5