[English] 日本語
 Yorodumi
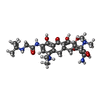
Yorodumi- PDB-8rri: Human mitochondrial ribosome in complex with antibiotic tigecycline -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8rri | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human mitochondrial ribosome in complex with antibiotic tigecycline | ||||||||||||||||||
 Components Components |
| ||||||||||||||||||
 Keywords Keywords | RIBOSOME / antibiotics / immunometabolism / mitochondrial ribosomes / tetracyclines / T cells. | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationrRNA import into mitochondrion / mitochondrial translational termination / mitochondrial ribosome assembly / mitochondrial translational elongation / Mitochondrial translation elongation / Mitochondrial translation termination / translation release factor activity, codon nonspecific / Mitochondrial translation initiation / translation release factor activity / negative regulation of mitotic nuclear division ...rRNA import into mitochondrion / mitochondrial translational termination / mitochondrial ribosome assembly / mitochondrial translational elongation / Mitochondrial translation elongation / Mitochondrial translation termination / translation release factor activity, codon nonspecific / Mitochondrial translation initiation / translation release factor activity / negative regulation of mitotic nuclear division / mitochondrial large ribosomal subunit / peptidyl-tRNA hydrolase / mitochondrial ribosome / Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters / mitochondrial small ribosomal subunit / peptidyl-tRNA hydrolase activity / mitochondrial translation / positive regulation of proteolysis / ribosomal small subunit binding / anatomical structure morphogenesis / RNA processing / rescue of stalled ribosome / Mitochondrial protein degradation / cellular response to leukemia inhibitory factor / apoptotic signaling pathway / fibrillar center / cell junction / regulation of translation / double-stranded RNA binding / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / large ribosomal subunit rRNA binding / small ribosomal subunit rRNA binding / endonuclease activity / nuclear membrane / tRNA binding / cell population proliferation / mitochondrial inner membrane / negative regulation of translation / rRNA binding / nuclear body / structural constituent of ribosome / ribosome / mitochondrial matrix / translation / protein domain specific binding / ribonucleoprotein complex / nucleotide binding / intracellular membrane-bounded organelle / mRNA binding / apoptotic process / GTP binding / nucleolus / mitochondrion / extracellular space / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.4 Å | ||||||||||||||||||
 Authors Authors | Khawaja, A. / Sing, V. / Nguyen, M.D. / Rorbach, J. | ||||||||||||||||||
| Funding support |  Sweden, 1items Sweden, 1items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: T cell toxicity induced by tigecycline binding to the mitochondrial ribosome. Authors: Qiuya Shao / Anas Khawaja / Minh Duc Nguyen / Vivek Singh / Jingdian Zhang / Yong Liu / Joel Nordin / Monika Adori / C Axel Innis / Xaquin Castro Dopico / Joanna Rorbach /      Abstract: Tetracyclines are essential bacterial protein synthesis inhibitors under continual development to combat antibiotic resistance yet suffer from unwanted side effects. Mitoribosomes - responsible for ...Tetracyclines are essential bacterial protein synthesis inhibitors under continual development to combat antibiotic resistance yet suffer from unwanted side effects. Mitoribosomes - responsible for generating oxidative phosphorylation (OXPHOS) subunits - share structural similarities with bacterial machinery and may suffer from cross-reactivity. Since lymphocytes rely upon OXPHOS upregulation to establish immunity, we set out to assess the impact of ribosome-targeting antibiotics on human T cells. We find tigecycline, a third-generation tetracycline, to be the most cytotoxic compound tested. In vitro, 5-10 μM tigecycline inhibits mitochondrial but not cytosolic translation, mitochondrial complex I, III and IV expression, and curtails the activation and expansion of unique T cell subsets. By cryo-EM, we find tigecycline to occupy three sites on T cell mitoribosomes. In addition to the conserved A-site found in bacteria, tigecycline also attaches to the peptidyl transferase center of the large subunit. Furthermore, a third, distinct binding site on the large subunit, aligns with helices analogous to those in bacteria, albeit lacking methylation in humans. The data provide a mechanism to explain part of the anti-inflammatory effects of these drugs and inform antibiotic design. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8rri.cif.gz 8rri.cif.gz | 7.4 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8rri.ent.gz pdb8rri.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8rri.json.gz 8rri.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8rri_validation.pdf.gz 8rri_validation.pdf.gz | 1.7 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8rri_full_validation.pdf.gz 8rri_full_validation.pdf.gz | 1.8 MB | Display | |
| Data in XML |  8rri_validation.xml.gz 8rri_validation.xml.gz | 345.1 KB | Display | |
| Data in CIF |  8rri_validation.cif.gz 8rri_validation.cif.gz | 614 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rr/8rri https://data.pdbj.org/pub/pdb/validation_reports/rr/8rri ftp://data.pdbj.org/pub/pdb/validation_reports/rr/8rri ftp://data.pdbj.org/pub/pdb/validation_reports/rr/8rri | HTTPS FTP |
-Related structure data
| Related structure data |  19460MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-RNA chain , 6 types, 6 molecules BAyAAAAzAx
| #1: RNA chain | Mass: 22989.752 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
|---|---|
| #82: RNA chain | Mass: 16669.957 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
| #83: RNA chain | Mass: 306218.312 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: GenBank: 1858624182 Homo sapiens (human) / References: GenBank: 1858624182 |
| #84: RNA chain | Mass: 499786.438 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
| #85: RNA chain | Mass: 10230.085 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
| #86: RNA chain | Mass: 22353.307 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) Homo sapiens (human) |
+39S ribosomal protein ... , 44 types, 44 molecules DEFHJLMNOPQRSTVWXYZ01234z56789...
-Large ribosomal subunit protein ... , 3 types, 3 molecules KUk
| #7: Protein | Mass: 20617.828 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q9BYD1 Homo sapiens (human) / References: UniProt: Q9BYD1 |
|---|---|
| #17: Protein | Mass: 17702.029 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q16540 Homo sapiens (human) / References: UniProt: Q16540 |
| #44: Protein | Mass: 12020.701 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q96EL3 Homo sapiens (human) / References: UniProt: Q96EL3 |
-Protein , 5 types, 5 molecules opqA3A4
| #47: Protein | Mass: 12292.333 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q9BQC6 Homo sapiens (human) / References: UniProt: Q9BQC6 |
|---|---|
| #48: Protein | Mass: 23674.203 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q14197, peptidyl-tRNA hydrolase Homo sapiens (human) / References: UniProt: Q14197, peptidyl-tRNA hydrolase |
| #49: Protein | Mass: 25426.895 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q8TAE8 Homo sapiens (human) / References: UniProt: Q8TAE8 |
| #80: Protein | Mass: 22395.326 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q9NWT8 Homo sapiens (human) / References: UniProt: Q9NWT8 |
| #81: Protein | Mass: 78648.547 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q96EY7 Homo sapiens (human) / References: UniProt: Q96EY7 |
+28S ribosomal protein ... , 25 types, 25 molecules ABACADAEAFAGAHAIAJAKALAMANAOAPARASAUAVAWAXAYAZA0A1
-Small ribosomal subunit protein ... , 3 types, 3 molecules AQATA2
| #67: Protein | Mass: 10659.494 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: P82921 Homo sapiens (human) / References: UniProt: P82921 |
|---|---|
| #70: Protein | Mass: 19717.045 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: P82663 Homo sapiens (human) / References: UniProt: P82663 |
| #79: Protein | Mass: 13393.661 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q96BP2 Homo sapiens (human) / References: UniProt: Q96BP2 |
-Non-polymers , 8 types, 1089 molecules 













| #87: Chemical | ChemComp-VAL / | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #88: Chemical | ChemComp-MG / #89: Chemical | #90: Chemical | ChemComp-ATP / | #91: Chemical | ChemComp-GDP / | #92: Chemical | #93: Chemical | ChemComp-K / #94: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human mitochondrial ribosome in complex with antibiotic tigecycline Type: CELL Entity ID: #2-#49, #51-#65, #67-#69, #71-#73, #75-#82, #85-#86 Source: NATURAL |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1600 nm / Nominal defocus min: 400 nm |
| Image recording | Electron dose: 45 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Version: 1.20.1_4487: / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 266550 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj
















































