[English] 日本語
 Yorodumi
Yorodumi- PDB-4b2q: Model of the yeast F1Fo-ATP synthase dimer based on subtomogram a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4b2q | ||||||
|---|---|---|---|---|---|---|---|
| Title | Model of the yeast F1Fo-ATP synthase dimer based on subtomogram average | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE / SUBTOMOGRAM AVERAGE | ||||||
| Function / homology |  Function and homology information Function and homology informationFormation of ATP by chemiosmotic coupling / Cristae formation / Mitochondrial protein degradation / Mitochondrial protein degradation / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / mitochondrial nucleoid / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism ...Formation of ATP by chemiosmotic coupling / Cristae formation / Mitochondrial protein degradation / Mitochondrial protein degradation / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / mitochondrial nucleoid / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / mitochondrial intermembrane space / mitochondrial inner membrane / lipid binding / ATP hydrolysis activity / mitochondrion / ATP binding / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |   | ||||||
| Method | ELECTRON MICROSCOPY / electron tomography / cryo EM / Resolution: 37 Å | ||||||
 Authors Authors | Davies, K.M. / Kuehlbrandt, W. | ||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2012 Journal: Proc Natl Acad Sci U S A / Year: 2012Title: Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Authors: Karen M Davies / Claudio Anselmi / Ilka Wittig / José D Faraldo-Gómez / Werner Kühlbrandt /  Abstract: We used electron cryotomography of mitochondrial membranes from wild-type and mutant Saccharomyces cerevisiae to investigate the structure and organization of ATP synthase dimers in situ. Subtomogram ...We used electron cryotomography of mitochondrial membranes from wild-type and mutant Saccharomyces cerevisiae to investigate the structure and organization of ATP synthase dimers in situ. Subtomogram averaging of the dimers to 3.7 nm resolution revealed a V-shaped structure of twofold symmetry, with an angle of 86° between monomers. The central and peripheral stalks are well resolved. The monomers interact within the membrane at the base of the peripheral stalks. In wild-type mitochondria ATP synthase dimers are found in rows along the highly curved cristae ridges, and appear to be crucial for membrane morphology. Strains deficient in the dimer-specific subunits e and g or the first transmembrane helix of subunit 4 lack both dimers and lamellar cristae. Instead, cristae are either absent or balloon-shaped, with ATP synthase monomers distributed randomly in the membrane. Computer simulations indicate that isolated dimers induce a plastic deformation in the lipid bilayer, which is partially relieved by their side-by-side association. We propose that the assembly of ATP synthase dimer rows is driven by the reduction in the membrane elastic energy, rather than by direct protein contacts, and that the dimer rows enable the formation of highly curved ridges in mitochondrial cristae. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "BA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "CA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "BA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "CA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4b2q.cif.gz 4b2q.cif.gz | 1.5 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4b2q.ent.gz pdb4b2q.ent.gz | 1.2 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4b2q.json.gz 4b2q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b2/4b2q https://data.pdbj.org/pub/pdb/validation_reports/b2/4b2q ftp://data.pdbj.org/pub/pdb/validation_reports/b2/4b2q ftp://data.pdbj.org/pub/pdb/validation_reports/b2/4b2q | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 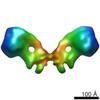 2161MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-ATP SYNTHASE SUBUNIT ... , 11 types, 44 molecules ACacBbDdEFefGgHhIiJKLMNOPQRSjk...
| #1: Protein | Mass: 52376.539 Da / Num. of mol.: 4 / Fragment: RESIDUES 61-545 / Source method: isolated from a natural source / Source: (natural)  #2: Protein | Mass: 52447.617 Da / Num. of mol.: 2 / Fragment: RESIDUES 60-545 / Source method: isolated from a natural source / Source: (natural)  #3: Protein | Mass: 50438.348 Da / Num. of mol.: 2 / Fragment: RESIDUES 39-508 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P00830, H+-transporting two-sector ATPase #4: Protein | Mass: 50752.641 Da / Num. of mol.: 4 / Fragment: RESIDUES 39-511 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P00830, H+-transporting two-sector ATPase #5: Protein | Mass: 30657.160 Da / Num. of mol.: 2 / Fragment: RESIDUES 34-311 / Source method: isolated from a natural source / Source: (natural)  #6: Protein | Mass: 14080.876 Da / Num. of mol.: 2 / Fragment: RESIDUES 29-160 / Source method: isolated from a natural source / Source: (natural)  #7: Protein | Mass: 6388.076 Da / Num. of mol.: 2 / Fragment: RESIDUES 2-60 / Source method: isolated from a natural source / Source: (natural)  #8: Protein | Mass: 7762.375 Da / Num. of mol.: 20 / Source method: isolated from a natural source / Source: (natural)  #9: Protein | Mass: 15408.768 Da / Num. of mol.: 2 / Fragment: RESIDUES 121-249 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P13619, H+-transporting two-sector ATPase #10: Protein | Mass: 13768.688 Da / Num. of mol.: 2 / Fragment: RESIDUES 5-124 / Source method: isolated from a natural source / Source: (natural)  #12: Protein | Mass: 13240.513 Da / Num. of mol.: 2 / Fragment: RESIDUES 24-143 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P13621, H+-transporting two-sector ATPase |
|---|
-Protein , 1 types, 2 molecules Vv
| #11: Protein | Mass: 7829.806 Da / Num. of mol.: 2 / Fragment: RESIDUES 36-101 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Non-polymers , 3 types, 20 molecules 




| #13: Chemical | ChemComp-ATP / #14: Chemical | ChemComp-MG / #15: Chemical | ChemComp-ADP / |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: CELL / 3D reconstruction method: electron tomography |
- Sample preparation
Sample preparation
| Component | Name: ATP SYNTHASE DIMER FROM MITOCHONDRIA OF SACCHAROMYCES CEREVISIAE Type: CELL |
|---|---|
| Buffer solution | Name: 250MM TREHALOSE 10NM TRIS- HCL PH7.4 / pH: 7.4 / Details: 250MM TREHALOSE 10NM TRIS- HCL PH7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: HOLEY CARBON |
| Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE Details: VITRIFICATION 1 -- CRYOGEN- ETHANE, TEMPERATURE- 100, INSTRUMENT- HOMEMADE PLUNGER, METHOD- SINGLE SIDE MANUAL BLOTTING FOR 5 SECONDS., |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI POLARA 300 / Date: Mar 11, 2009 |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 41000 X / Calibrated magnification: 24500 X / Nominal defocus max: 7500 nm / Nominal defocus min: 6500 nm / Cs: 2 mm |
| Specimen holder | Temperature: 80 K / Tilt angle max: 60 ° / Tilt angle min: -50 ° |
| Image recording | Electron dose: 160 e/Å2 / Film or detector model: GATAN ULTRASCAN 1000 (2k x 2k) |
| Image scans | Num. digital images: 75 |
| Radiation wavelength | Relative weight: 1 |
- Processing
Processing
| EM software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||
| 3D reconstruction | Method: WEIGHTED BACK-PROJECTION / Resolution: 37 Å / Num. of particles: 121 / Actual pixel size: 5.76 Å Details: MODEL BASED ON PDBS 2WPD,2CLY AND 2BO5. SUBMISSION BASED ON EXPERIMENTAL DATA FROM EMDB EMD-2161. (DEPOSITION ID: 10941). Symmetry type: POINT | ||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL / Details: METHOD--RIGID BODY REFINEMENT PROTOCOL--X-RAY | ||||||||||||
| Atomic model building | PDB-ID: 2WPD Accession code: 2WPD / Source name: PDB / Type: experimental model | ||||||||||||
| Refinement | Highest resolution: 37 Å | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 37 Å
|
 Movie
Movie Controller
Controller









 PDBj
PDBj





