[English] 日本語
 Yorodumi
Yorodumi- EMDB-49933: Cryo-EM structure of the glycosyltransferase GtrB (tetramer volume) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the glycosyltransferase GtrB (tetramer volume) | |||||||||
 Map data Map data | structure of the glycosyltransferase GtrB (tetramer volume) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glycosyltransferase / polyisoprenyl / MEMBRANE PROTEIN | |||||||||
| Function / homology | Transferases; Glycosyltransferases / : / Glycosyltransferase 2-like / Glycosyl transferase family 2 / glycosyltransferase activity / Nucleotide-diphospho-sugar transferases / plasma membrane / Uncharacterized glycosyltransferase sll0501 Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.19 Å | |||||||||
 Authors Authors | Morgan RT / Motta S / Gil-Iturbe E / di Muccio G / Bhattacharjee B / Romagnoli A / Anwar MT / Mishra B / Ashraf K / Bang I ...Morgan RT / Motta S / Gil-Iturbe E / di Muccio G / Bhattacharjee B / Romagnoli A / Anwar MT / Mishra B / Ashraf K / Bang I / di Marino D / Lowary TL / Quick M / Petrou VI / Stowell MHB / Nygaard R / Mancia F | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Mechanistic snapshots of lipid-linked sugar transfer. Authors: Ryan T Morgan / Stefano Motta / Eva Gil-Iturbe / Biddut Bhattacharjee / Mohammad T Anwar / Giovanni Di Muccio / Alice Romagnoli / Bedangshu Mishra / Khuram U Ashraf / Injin Bang / Daniele Di ...Authors: Ryan T Morgan / Stefano Motta / Eva Gil-Iturbe / Biddut Bhattacharjee / Mohammad T Anwar / Giovanni Di Muccio / Alice Romagnoli / Bedangshu Mishra / Khuram U Ashraf / Injin Bang / Daniele Di Marino / Todd L Lowary / Matthias Quick / Vasileios I Petrou / Michael H B Stowell / Rie Nygaard / Filippo Mancia /    Abstract: Enzymes undergo dynamic conformational changes during catalysis, yet conventional high-resolution structural methods typically capture only the most stable states. Here, we address this gap using ...Enzymes undergo dynamic conformational changes during catalysis, yet conventional high-resolution structural methods typically capture only the most stable states. Here, we address this gap using rapid UV photolysis of a chemically caged substrate with cryogenic time-resolved electron microscopy (cryo-TREM). We elucidate the catalytic mechanism of GtrB, a membrane-bound glycosyltransferase that transfers glucose from UDP-glucose to the lipid carrier undecaprenyl phosphate. We visualized how GtrB, which has an active site ~15 Å from the membrane, transitions during the catalytic cycle to move each substrate in proximity for catalysis. From a single dataset, we resolved distinct conformational states: the initial substrate-bound state, a catalytically poised intermediate, and the product-bound state. Through molecular dynamics simulations and biochemical analyses, we identify coordinated movements within the active site that drive catalysis. These findings provide a molecular framework for understanding how glycosyltransferases function and highlight a broadly applicable strategy for capturing dynamic enzymatic states in native-like environments. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_49933.map.gz emd_49933.map.gz | 97.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-49933-v30.xml emd-49933-v30.xml emd-49933.xml emd-49933.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_49933_fsc.xml emd_49933_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_49933.png emd_49933.png | 58.3 KB | ||
| Masks |  emd_49933_msk_1.map emd_49933_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-49933.cif.gz emd-49933.cif.gz | 6.7 KB | ||
| Others |  emd_49933_half_map_1.map.gz emd_49933_half_map_1.map.gz emd_49933_half_map_2.map.gz emd_49933_half_map_2.map.gz | 95.3 MB 95.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-49933 http://ftp.pdbj.org/pub/emdb/structures/EMD-49933 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-49933 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-49933 | HTTPS FTP |
-Related structure data
| Related structure data |  9nyfMC  9nycC  9nydC  9nyeC  9nykC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_49933.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_49933.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | structure of the glycosyltransferase GtrB (tetramer volume) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_49933_msk_1.map emd_49933_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_49933_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map A
| File | emd_49933_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GtrB
| Entire | Name: GtrB |
|---|---|
| Components |
|
-Supramolecule #1: GtrB
| Supramolecule | Name: GtrB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: Map originates from tetramer particles |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 146.673 KDa |
-Macromolecule #1: Glycosyltransferase sll0501
| Macromolecule | Name: Glycosyltransferase sll0501 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: Transferases; Glycosyltransferases |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.872016 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHHHH HSSGVDLGTE NLYFQSNATI ELSIVIPMYN EEDNLEHLFA RLLEVLTPLK ITYEIICVND GSKDKTLKQL IDCYQSNRQ IKIVNLSRNF GKEIALSAGI DYAQGNAVIP IDADLQDPPE LIHELVDKWR EGYDIVYATR RSRQGETWVK Q FTAKMFYK ...String: MHHHHHHHHH HSSGVDLGTE NLYFQSNATI ELSIVIPMYN EEDNLEHLFA RLLEVLTPLK ITYEIICVND GSKDKTLKQL IDCYQSNRQ IKIVNLSRNF GKEIALSAGI DYAQGNAVIP IDADLQDPPE LIHELVDKWR EGYDIVYATR RSRQGETWVK Q FTAKMFYK VIGRMTEIKI PPNTGDFRLM DRKVVNAIKQ LPERTRFMKG LFAWVGYRQT FVLFDREPRF QGQTKWNYWK LW NFALDGI FSFSLLPLKV WTYLGSIISL LSLAYASFLI LKTITLGVDV PGYASLMVAI LFLGGVQLIS LGVIGEYLGR VYE EVKARP LYLVSDLWGL EYLPLEKLN UniProtKB: Uncharacterized glycosyltransferase sll0501 |
-Macromolecule #2: URIDINE-5'-DIPHOSPHATE-GLUCOSE
| Macromolecule | Name: URIDINE-5'-DIPHOSPHATE-GLUCOSE / type: ligand / ID: 2 / Number of copies: 4 / Formula: UPG |
|---|---|
| Molecular weight | Theoretical: 566.302 Da |
| Chemical component information | 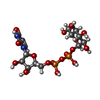 ChemComp-UPG: |
-Macromolecule #3: [(2~{E},6~{E},10~{E},14~{E},18~{Z},22~{E},26~{Z},30~{E},34~{E},38...
| Macromolecule | Name: [(2~{E},6~{E},10~{E},14~{E},18~{Z},22~{E},26~{Z},30~{E},34~{E},38~{E})-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaenyl] dihydrogen phosphate type: ligand / ID: 3 / Number of copies: 4 / Formula: 5TR |
|---|---|
| Molecular weight | Theoretical: 847.282 Da |
| Chemical component information | 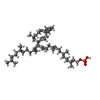 ChemComp-5TR: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.22 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Vitrification carried out under UV exposure for 17 ms. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 9219 / Average exposure time: 2.5 sec. / Average electron dose: 58.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.5 µm / Calibrated defocus min: 1.5 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)














































