+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human liver phosphofructokinase-1 in the R-state conformation | |||||||||
 Map data Map data | PFKL R-state tetramer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | PFK / glycolysis / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology information6-phosphofructokinase complex / 6-phosphofructokinase / fructose binding / 6-phosphofructokinase activity / fructose-6-phosphate binding / fructose 1,6-bisphosphate metabolic process / fructose 6-phosphate metabolic process / canonical glycolysis / Glycolysis / response to glucose ...6-phosphofructokinase complex / 6-phosphofructokinase / fructose binding / 6-phosphofructokinase activity / fructose-6-phosphate binding / fructose 1,6-bisphosphate metabolic process / fructose 6-phosphate metabolic process / canonical glycolysis / Glycolysis / response to glucose / glycolytic process / negative regulation of insulin secretion / kinase binding / secretory granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation / extracellular exosome / extracellular region / ATP binding / metal ion binding / identical protein binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Lynch EM / Kollman JM / Webb BA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for allosteric regulation of human phosphofructokinase-1. Authors: Eric M Lynch / Heather Hansen / Lauren Salay / Madison Cooper / Stepan Timr / Justin M Kollman / Bradley A Webb /   Abstract: Phosphofructokinase-1 (PFK1) catalyzes the rate-limiting step of glycolysis, committing glucose to conversion into cellular energy. PFK1 is highly regulated to respond to the changing energy needs of ...Phosphofructokinase-1 (PFK1) catalyzes the rate-limiting step of glycolysis, committing glucose to conversion into cellular energy. PFK1 is highly regulated to respond to the changing energy needs of the cell. In bacteria, the structural basis of PFK1 regulation is a textbook example of allostery; molecular signals of low and high cellular energy promote transition between an active R-state and inactive T-state conformation, respectively. Little is known, however, about the structural basis for regulation of eukaryotic PFK1. Here, we determine structures of the human liver isoform of PFK1 (PFKL) in the R- and T-state by cryoEM, providing insight into eukaryotic PFK1 allosteric regulatory mechanisms. The T-state structure reveals conformational differences between the bacterial and eukaryotic enzyme, the mechanisms of allosteric inhibition by ATP binding at multiple sites, and an autoinhibitory role of the C-terminus in stabilizing the T-state. We also determine structures of PFKL filaments that define the mechanism of higher-order assembly and demonstrate that these structures are necessary for higher-order assembly of PFKL in cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43747.map.gz emd_43747.map.gz | 25.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43747-v30.xml emd-43747-v30.xml emd-43747.xml emd-43747.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43747.png emd_43747.png | 88.1 KB | ||
| Filedesc metadata |  emd-43747.cif.gz emd-43747.cif.gz | 6.2 KB | ||
| Others |  emd_43747_half_map_1.map.gz emd_43747_half_map_1.map.gz emd_43747_half_map_2.map.gz emd_43747_half_map_2.map.gz | 226.1 MB 226.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43747 http://ftp.pdbj.org/pub/emdb/structures/EMD-43747 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43747 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43747 | HTTPS FTP |
-Related structure data
| Related structure data |  8w2gMC  8w2hC  8w2iC  8w2jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43747.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43747.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | PFKL R-state tetramer | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.843 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_43747_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_43747_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : PFKL tetramer
| Entire | Name: PFKL tetramer |
|---|---|
| Components |
|
-Supramolecule #1: PFKL tetramer
| Supramolecule | Name: PFKL tetramer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: ATP-dependent 6-phosphofructokinase, liver type
| Macromolecule | Name: ATP-dependent 6-phosphofructokinase, liver type / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: 6-phosphofructokinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 85.120375 KDa |
| Recombinant expression | Organism:  unidentified baculovirus unidentified baculovirus |
| Sequence | String: MAAVDLEKLR ASGAGKAIGV LTSGGDAQGM NAAVRAVTRM GIYVGAKVFL IYEGYEGLVE GGENIKQANW LSVSNIIQLG GTIIGSARC KAFTTREGRR AAAYNLVQHG ITNLCVIGGD GSLTGANIFR SEWGSLLEEL VAEGKISETT ARTYSHLNIA G LVGSIDND ...String: MAAVDLEKLR ASGAGKAIGV LTSGGDAQGM NAAVRAVTRM GIYVGAKVFL IYEGYEGLVE GGENIKQANW LSVSNIIQLG GTIIGSARC KAFTTREGRR AAAYNLVQHG ITNLCVIGGD GSLTGANIFR SEWGSLLEEL VAEGKISETT ARTYSHLNIA G LVGSIDND FCGTDMTIGT DSALHRIMEV IDAITTTAQS HQRTFVLEVM GRHCGYLALV SALASGADWL FIPEAPPEDG WE NFMCERL GETRSRGSRL NIIIIAEGAI DRNGKPISSS YVKDLVVQRL GFDTRVTVLG HVQRGGTPSA FDRILSSKMG MEA VMALLE ATPDTPACVV TLSGNQSVRL PLMECVQMTK EVQKAMDDKR FDEATQLRGG SFENNWNIYK LLAHQKPPKE KSNF SLAIL NVGAPAAGMN AAVRSAVRTG ISHGHTVYVV HDGFEGLAKG QVQEVGWHDV AGWLGRGGSM LGTKRTLPKG QLESI VENI RIYGIHALLV VGGFEAYEGV LQLVEARGRY EELCIVMCVI PATISNNVPG TDFSLGSDTA VNAAMESCDR IKQSAS GTK RRVFIVETMG GYCGYLATVT GIAVGADAAY VFEDPFNIHD LKVNVEHMTE KMKTDIQRGL VLRNEKCHDY YTTEFLY NL YSSEGKGVFD CRTNVLGHLQ QGGAPTPFDR NYGTKLGVKA MLWLSEKLRE VYRKGRVFAN APDSACVIGL KKKAVAFS P VTELKKDTDF EHRMPREQWW LSLRLMLKML AQYRISMAAY VSGELEHVTR RTLSMDKGF UniProtKB: ATP-dependent 6-phosphofructokinase, liver type |
-Macromolecule #2: 6-O-phosphono-beta-D-fructofuranose
| Macromolecule | Name: 6-O-phosphono-beta-D-fructofuranose / type: ligand / ID: 2 / Number of copies: 4 / Formula: F6P |
|---|---|
| Molecular weight | Theoretical: 260.136 Da |
| Chemical component information |  ChemComp-F6P: |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 12 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: 1,6-di-O-phosphono-beta-D-fructofuranose
| Macromolecule | Name: 1,6-di-O-phosphono-beta-D-fructofuranose / type: ligand / ID: 4 / Number of copies: 4 / Formula: FBP |
|---|---|
| Molecular weight | Theoretical: 340.116 Da |
| Chemical component information | 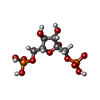 ChemComp-FBP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 63.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: Modeller / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-8w2g: |
 Movie
Movie Controller
Controller









 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)




































