+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
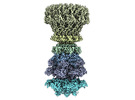
| Title | Cryo-EM of neck of bacteriophage Chi | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Flagellotropic bacteriophage / Siphophage / Neck / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationviral portal complex / virion assembly / viral life cycle / symbiont entry into host cell / structural molecule activity / DNA binding Similarity search - Function | |||||||||
| Biological species |  Chivirus chi Chivirus chi | |||||||||
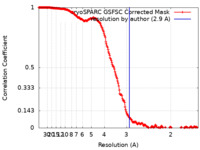
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Sonani RR / Esteves NC / Scharf BE / Egelman EH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Cryo-EM structure of flagellotropic bacteriophage Chi. Authors: Ravi R Sonani / Nathaniel C Esteves / Birgit E Scharf / Edward H Egelman /  Abstract: The flagellotropic bacteriophage χ (Chi) infects bacteria via the flagellar filament. Despite years of study, its structural architecture remains partly characterized. Through cryo-EM, we unveil ...The flagellotropic bacteriophage χ (Chi) infects bacteria via the flagellar filament. Despite years of study, its structural architecture remains partly characterized. Through cryo-EM, we unveil χ's nearly complete structure, encompassing capsid, neck, tail, and tail tip. While the capsid and tail resemble phage YSD1, the neck and tail tip reveal new proteins and their arrangement. The neck shows a unique conformation of the tail tube protein, forming a socket-like structure for attachment to the neck. The tail tip comprises four proteins, including distal tail protein (DTP), two baseplate hub proteins (BH1P and BH2P), and tail tip assembly protein (TAP) exhibiting minimal organization compared to other siphophages. Deviating from the consensus in other siphophages, DTP in χ forms a trimeric assembly, reducing tail symmetry from 6-fold to 3-fold at the tip. These findings illuminate the previously unexplored structural organization of χ's neck and tail tip. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43243.map.gz emd_43243.map.gz | 770.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43243-v30.xml emd-43243-v30.xml emd-43243.xml emd-43243.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43243_fsc.xml emd_43243_fsc.xml | 19.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_43243.png emd_43243.png | 78.6 KB | ||
| Filedesc metadata |  emd-43243.cif.gz emd-43243.cif.gz | 5.9 KB | ||
| Others |  emd_43243_half_map_1.map.gz emd_43243_half_map_1.map.gz emd_43243_half_map_2.map.gz emd_43243_half_map_2.map.gz | 764.1 MB 764.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43243 http://ftp.pdbj.org/pub/emdb/structures/EMD-43243 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43243 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43243 | HTTPS FTP |
-Validation report
| Summary document |  emd_43243_validation.pdf.gz emd_43243_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43243_full_validation.pdf.gz emd_43243_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_43243_validation.xml.gz emd_43243_validation.xml.gz | 28.9 KB | Display | |
| Data in CIF |  emd_43243_validation.cif.gz emd_43243_validation.cif.gz | 38.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43243 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43243 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43243 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43243 | HTTPS FTP |
-Related structure data
| Related structure data |  8vhxMC  8vjaC  8vjhC  8vjiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43243.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43243.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||
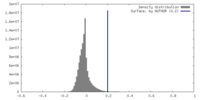
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_43243_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
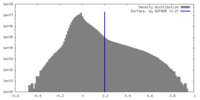
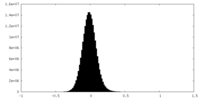
| Density Histograms |
-Half map: #1
| File | emd_43243_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
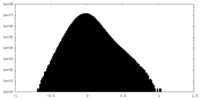
| Density Histograms |
- Sample components
Sample components
-Entire : Chivirus chi
| Entire | Name:  Chivirus chi Chivirus chi |
|---|---|
| Components |
|
-Supramolecule #1: Chivirus chi
| Supramolecule | Name: Chivirus chi / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 1541887 / Sci species name: Chivirus chi / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Neck 1
| Macromolecule | Name: Neck 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chivirus chi Chivirus chi |
| Molecular weight | Theoretical: 9.431688 KDa |
| Sequence | String: MTPEECRAQY RLMLKEAMDA YHQLNLGGSV RVVVDQNSER VEYTAANRQS LWAYIVRLQN AINSDNPCAA FMGLPSSPAG FLFP UniProtKB: Uncharacterized protein |
-Macromolecule #2: Tail terminator
| Macromolecule | Name: Tail terminator / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chivirus chi Chivirus chi |
| Molecular weight | Theoretical: 18.804584 KDa |
| Sequence | String: MSQRLDILKA LTAHLEQITI ANGYAYDLKG KVYRGRDRFG ADFTSRLPIV SILEAKATDY GSFANEEQTV RMDDWVLLVQ GWVKDDPRN PTDPAYELLA EVEKRLAMLV AKDEQGQPMY PALYRLGGKI AKLTLAQPVV RPPEDGLSDT AFFFLPVRVG L KVDIRNP UniProtKB: Transposase |
-Macromolecule #3: Portal
| Macromolecule | Name: Portal / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chivirus chi Chivirus chi |
| Molecular weight | Theoretical: 62.333262 KDa |
| Sequence | String: MTEKKRSTTQ RAKKAAKTAD VATLDATPQN PSALGGGLEG AERNTREMFR WTPAIISPDQ QIAQDGTLAL SRAQDIVQND GYAFGAVAI HRDSVVGSQY KLNSKPNSLV LGAPEGWAEE FQEVVEARFN MVAESPENWF DARRMNTLTG LVRLAVGGFI M TGEVLASC ...String: MTEKKRSTTQ RAKKAAKTAD VATLDATPQN PSALGGGLEG AERNTREMFR WTPAIISPDQ QIAQDGTLAL SRAQDIVQND GYAFGAVAI HRDSVVGSQY KLNSKPNSLV LGAPEGWAEE FQEVVEARFN MVAESPENWF DARRMNTLTG LVRLAVGGFI M TGEVLASC EWMKPNGTRM QRRPFGTAIQ MISPYRLSNP DNIMDDKYLR SGVKLDEMGA PIGYWLRKAF PGDPTDLEQW RW EYQPARF DWGRRRMIHI IEALLPGQTR GISEMVAALK QMKMTRNFQE VTLQNAIVNA TYAAAIESEL PSDVVFNQMG MGQ TPFGDI LKTYMGSLAE YIAGSKNIAI DGAKIPHLFP GTKLKMQPAG TPGGVGTDYE ESLLRNIAAS LGLSYEQFSR DYTK TNYSS ARASMAETWK YMESRKKLVA DRFASMIYTL WLEEEVNAGN VPLPPGFTWR DFYDPMKRDA LCNAEWIGAS RGQID EKKE TEAAILRIKN GLSTYEAEIA RLGGDFREVF KQRAREEGII KDLGLDFSGK MVEGTEASGS TGSTGSDNNN EEDTKE UniProtKB: Portal protein |
-Macromolecule #4: Neck 2
| Macromolecule | Name: Neck 2 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chivirus chi Chivirus chi |
| Molecular weight | Theoretical: 13.39921 KDa |
| Sequence | String: MASNFAAIKA KARRDVHASL SVPARYENYS QDVIVEDLSV RWHNKIAIMG DLENGGYANI VEGIERIIFT REELAVKGVV LSEGDSIIM TAEGYENARL VLKTQEPIVG PVEVVWQVAR AD UniProtKB: Uncharacterized protein |
-Macromolecule #5: Tail Tube
| Macromolecule | Name: Tail Tube / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chivirus chi Chivirus chi |
| Molecular weight | Theoretical: 40.375363 KDa |
| Sequence | String: MNDNYQNNYV VGRGTVYFDR FQDGTNRKTG EMYFGNTPEF TINTDSETLD HYSSDHGMRV MDASVLLEAS QGGTFTCDNI NADNLALWF LGEVSNTTQT QQTDAKEVFN PIMRGRYYQL GTTDDNPTGV RGVTNFQMVK ADASIAISVG SGDITSIVGA T VVNPAGNY ...String: MNDNYQNNYV VGRGTVYFDR FQDGTNRKTG EMYFGNTPEF TINTDSETLD HYSSDHGMRV MDASVLLEAS QGGTFTCDNI NADNLALWF LGEVSNTTQT QQTDAKEVFN PIMRGRYYQL GTTDDNPTGV RGVTNFQMVK ADASIAISVG SGDITSIVGA T VVNPAGNY EIDLEAGRIY IEPDSTDLSG NVQIAVQYDV DAQKRTLVIG KSNMVYGALR MISDNPVGLN KNYYFPKVSI AP DGDYALK GDDWQVMSFT FKAMQLNNIT QRVYIDIVEA AAAVDPTAQR TIEITPASTT ATTGGAGVVC TVTVRDGTGT AVQ GDAVTF TTVAGATVTP NSATTGATGT ATTTVNRAAA GTATVTATLA NGKAATTGTI TFSAP UniProtKB: Major tail protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
| Details | Neck region of bacteriophage Chi |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)