+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Multidrug efflux pump EfpA from mycobacterium smegmatis | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | multidrug efflux pump / EfpA / Mycobacterium smegmatis / TRANSPORT PROTEIN | |||||||||
| Function / homology | Major facilitator superfamily / Major Facilitator Superfamily / Major facilitator superfamily domain / Major facilitator superfamily (MFS) profile. / transmembrane transporter activity / MFS transporter superfamily / plasma membrane / Putative MFS-type transporter EfpA Function and homology information Function and homology information | |||||||||
| Biological species |  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) | |||||||||
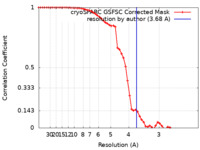
| Method | single particle reconstruction / cryo EM / Resolution: 3.68 Å | |||||||||
 Authors Authors | Wang S / Liao M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structures of the Mycobacterium tuberculosis efflux pump EfpA reveal the mechanisms of transport and inhibition. Authors: Shuhui Wang / Kun Wang / Kangkang Song / Zon Weng Lai / Pengfei Li / Dongying Li / Yajie Sun / Ye Mei / Chen Xu / Maofu Liao /   Abstract: As the first identified multidrug efflux pump in Mycobacterium tuberculosis (Mtb), EfpA is an essential protein and promising drug target. However, the functional and inhibitory mechanisms of EfpA ...As the first identified multidrug efflux pump in Mycobacterium tuberculosis (Mtb), EfpA is an essential protein and promising drug target. However, the functional and inhibitory mechanisms of EfpA are poorly understood. Here we report cryo-EM structures of EfpA in outward-open conformation, either bound to three endogenous lipids or the inhibitor BRD-8000.3. Three lipids inside EfpA span from the inner leaflet to the outer leaflet of the membrane. BRD-8000.3 occupies one lipid site at the level of inner membrane leaflet, competitively inhibiting lipid binding. EfpA resembles the related lysophospholipid transporter MFSD2A in both overall structure and lipid binding sites and may function as a lipid flippase. Combining AlphaFold-predicted EfpA structure, which is inward-open, we propose a complete conformational transition cycle for EfpA. Together, our results provide a structural and mechanistic foundation to comprehend EfpA function and develop EfpA-targeting anti-TB drugs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42205.map.gz emd_42205.map.gz | 15.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42205-v30.xml emd-42205-v30.xml emd-42205.xml emd-42205.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42205_fsc.xml emd_42205_fsc.xml | 5.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_42205.png emd_42205.png | 59.8 KB | ||
| Masks |  emd_42205_msk_1.map emd_42205_msk_1.map | 16.8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42205.cif.gz emd-42205.cif.gz | 5.5 KB | ||
| Others |  emd_42205_half_map_1.map.gz emd_42205_half_map_1.map.gz emd_42205_half_map_2.map.gz emd_42205_half_map_2.map.gz | 15.6 MB 15.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42205 http://ftp.pdbj.org/pub/emdb/structures/EMD-42205 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42205 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42205 | HTTPS FTP |
-Related structure data
| Related structure data |  8ufeMC  8ufdC  8wm5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42205.map.gz / Format: CCP4 / Size: 16.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42205.map.gz / Format: CCP4 / Size: 16.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42205_msk_1.map emd_42205_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_42205_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_42205_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : multidrug efflux pump MsEfpA
| Entire | Name: multidrug efflux pump MsEfpA |
|---|---|
| Components |
|
-Supramolecule #1: multidrug efflux pump MsEfpA
| Supramolecule | Name: multidrug efflux pump MsEfpA / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
-Macromolecule #1: Integral membrane efflux protein EfpA
| Macromolecule | Name: Integral membrane efflux protein EfpA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 48.39991 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: WGFLSAVIAI GGMQLLATMD STVAIVALPK IQDELSLSDA GRSWVITAYV LTFGGLMLLG GRLGDTIGRK RTFIVGVMLF TIASVLCGI AWNETTLVTA RLLQGVGAAI ASPTGLALVA TTFPKGPARN AATAVFGAMT AIGSVMGLVV GGALTEVSWR W AFLVNVPI ...String: WGFLSAVIAI GGMQLLATMD STVAIVALPK IQDELSLSDA GRSWVITAYV LTFGGLMLLG GRLGDTIGRK RTFIVGVMLF TIASVLCGI AWNETTLVTA RLLQGVGAAI ASPTGLALVA TTFPKGPARN AATAVFGAMT AIGSVMGLVV GGALTEVSWR W AFLVNVPI GLVMVYLART ALQETNRERM KLDAAGALLA TLACTAAVFA FTQGPESGWL APITLASGAA ALVFGLAFLI AE RNAENPV VPFALFRERN RVATFAAIFL AGGVLFTLTV LIGLYVQDIL GYSALRAGVG FIPFVIGMGI GLGAASQLVR SIP PRVLVI AGGILVLGAM IYGSTLHRGI PYFPNLVLPI TIGGIGIGTI VVPLTLSAIA GVNLDRIGPA SAIALMLQNL GGPL VLAVI QAVITSRTLF LGGTTGPVKA MNDEQIGALD AAYTYGLLWV AAVAVLVGAA ALFIGYTSQQ VAHAQ UniProtKB: Putative MFS-type transporter EfpA |
-Macromolecule #2: PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 2 / Number of copies: 1 / Formula: PTY |
|---|---|
| Molecular weight | Theoretical: 734.039 Da |
| Chemical component information |  ChemComp-PTY: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)