+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Cas8-HNH system at full R-loop state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | a protein complex / ANTIVIRAL PROTEIN/DNA/RNA / ANTIVIRAL PROTEIN-DNA-RNA complex | |||||||||
| Biological species |  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) | |||||||||
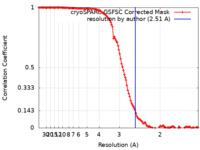
| Method | single particle reconstruction / cryo EM / Resolution: 2.51 Å | |||||||||
 Authors Authors | Zhang H / Zhu H / Li X / Liu Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2024 Journal: EMBO J / Year: 2024Title: Structural basis for the type I-F Cas8-HNH system. Authors: Xuzichao Li / Yanan Liu / Jie Han / Lingling Zhang / Zhikun Liu / Lin Wang / Shuqin Zhang / Qian Zhang / Pengyu Fu / Hang Yin / Hongtao Zhu / Heng Zhang /  Abstract: The Cas3 nuclease is utilized by canonical type I CRISPR-Cas systems for processive target DNA degradation, while a newly identified type I-F CRISPR variant employs an HNH nuclease domain from the ...The Cas3 nuclease is utilized by canonical type I CRISPR-Cas systems for processive target DNA degradation, while a newly identified type I-F CRISPR variant employs an HNH nuclease domain from the natural fusion Cas8-HNH protein for precise target cleavage both in vitro and in human cells. Here, we report multiple cryo-electron microscopy structures of the type I-F Cas8-HNH system at different functional states. The Cas8-HNH Cascade complex adopts an overall G-shaped architecture, with the HNH domain occupying the C-terminal helical bundle domain (HB) of the Cas8 protein in canonical type I systems. The Linker region connecting Cas8-NTD and HNH domains adopts a rigid conformation and interacts with the Cas7.6 subunit, enabling the HNH domain to be in a functional position. The full R-loop formation displaces the HNH domain away from the Cas6 subunit, thus activating the target DNA cleavage. Importantly, our results demonstrate that precise target cleavage is dictated by a C-terminal helix of the HNH domain. Together, our work not only delineates the structural basis for target recognition and activation of the type I-F Cas8-HNH system, but also guides further developments leveraging this system for precise DNA editing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39706.map.gz emd_39706.map.gz | 157.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39706-v30.xml emd-39706-v30.xml emd-39706.xml emd-39706.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39706_fsc.xml emd_39706_fsc.xml | 11.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_39706.png emd_39706.png | 60.3 KB | ||
| Filedesc metadata |  emd-39706.cif.gz emd-39706.cif.gz | 6.5 KB | ||
| Others |  emd_39706_half_map_1.map.gz emd_39706_half_map_1.map.gz emd_39706_half_map_2.map.gz emd_39706_half_map_2.map.gz | 165 MB 165 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39706 http://ftp.pdbj.org/pub/emdb/structures/EMD-39706 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39706 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39706 | HTTPS FTP |
-Validation report
| Summary document |  emd_39706_validation.pdf.gz emd_39706_validation.pdf.gz | 785.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39706_full_validation.pdf.gz emd_39706_full_validation.pdf.gz | 784.7 KB | Display | |
| Data in XML |  emd_39706_validation.xml.gz emd_39706_validation.xml.gz | 18.9 KB | Display | |
| Data in CIF |  emd_39706_validation.cif.gz emd_39706_validation.cif.gz | 24.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39706 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39706 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39706 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39706 | HTTPS FTP |
-Related structure data
| Related structure data |  8z0kMC  8z0lC  8zdyC  8znrC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_39706.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39706.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.75 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_39706_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_39706_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of Cas8-HNH system at full R-loop state
| Entire | Name: Cryo-EM structure of Cas8-HNH system at full R-loop state |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of Cas8-HNH system at full R-loop state
| Supramolecule | Name: Cryo-EM structure of Cas8-HNH system at full R-loop state type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
-Macromolecule #1: type I-F CRISPR-associated protein Csy3
| Macromolecule | Name: type I-F CRISPR-associated protein Csy3 / type: protein_or_peptide / ID: 1 Details: Molecular name from NCBI link:https://www.ncbi.nlm.nih.gov/protein/MBO6292766.1 Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 37.668945 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: TLKSRPENLS FARCLNTTEA KFWQTDFLKR HTFKLPLLIT DKAVLASKGH EMPPDKLEKE IMDPNPQKSQ SCTLSTECDT LRIDFGIKV LPVKESMYSC SDYNYRTAIY QKIDEYIAED GFLTLAKRYV NNIANARFLW RNRKGAEIIE TIVTIEDKEY P SFNSKSFN ...String: TLKSRPENLS FARCLNTTEA KFWQTDFLKR HTFKLPLLIT DKAVLASKGH EMPPDKLEKE IMDPNPQKSQ SCTLSTECDT LRIDFGIKV LPVKESMYSC SDYNYRTAIY QKIDEYIAED GFLTLAKRYV NNIANARFLW RNRKGAEIIE TIVTIEDKEY P SFNSKSFN LDTFVEDNAT INEIAQQIAD TFAGKREYLN IYVTCFVKIG CAMEVYPSQE MTFDDDDKGK KLFKFEGSAG MH SQKINNA LRTIDTWYPD YTTYEFPIPV ENYGAARSIG IPFRPDTKSF YKLIDRMILK NEDLPIEDKH YVMAILIRGG MFS KKQEK |
-Macromolecule #2: hypothetical protein J6N51_11000
| Macromolecule | Name: hypothetical protein J6N51_11000 / type: protein_or_peptide / ID: 2 Details: Molecular name from NCBI link:https://www.ncbi.nlm.nih.gov/protein/MBO6292763.1 Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 28.703135 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MMKGYILLEK VNIENANAFN NIIVGIPAIT SFLGFARALE RKLNAKEIAI RINGVGLEFH EYELKGYKNK RGQYVTSCPL PGSIPGQNE KKLDAHIMNQ AYIDLNMSFL LEVEGPHVDM STCKSIKSTM ETLRIAGGII RNYKKIRLID TLADIPYGYF L TLRQDNLN ...String: MMKGYILLEK VNIENANAFN NIIVGIPAIT SFLGFARALE RKLNAKEIAI RINGVGLEFH EYELKGYKNK RGQYVTSCPL PGSIPGQNE KKLDAHIMNQ AYIDLNMSFL LEVEGPHVDM STCKSIKSTM ETLRIAGGII RNYKKIRLID TLADIPYGYF L TLRQDNLN DAAGDDMLDK MIHALQQEDT LVPIAVGFKA LSEVGHVEGQ RDPEKDHCFV ESIFSLGGFE CSKILEDINS CL WRYKTEE GLYLCTII |
-Macromolecule #3: HNH endonuclease
| Macromolecule | Name: HNH endonuclease / type: protein_or_peptide / ID: 3 Details: Molecular name from NCBI link:https://www.ncbi.nlm.nih.gov/protein/MBO6292764.1 Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 39.339742 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLRNKILAAI SQKIPEEQKI NKYIEGLFQS IDKNHLATHV AKFTETNSPG NIGAYDILSS DMNCGYLDTA NAGWKEPDIV TNDAKYKRP QGFVAMEMSD GRTVMEHLQE DSAELRHEME ELTDKYDEIR DGILNMPSMQ PYRTNQFIKQ VFFPVGGSYH L LSILPSTV ...String: MLRNKILAAI SQKIPEEQKI NKYIEGLFQS IDKNHLATHV AKFTETNSPG NIGAYDILSS DMNCGYLDTA NAGWKEPDIV TNDAKYKRP QGFVAMEMSD GRTVMEHLQE DSAELRHEME ELTDKYDEIR DGILNMPSMQ PYRTNQFIKQ VFFPVGGSYH L LSILPSTV LNYEVSDRLY RSKIPKIRLR LLSSNAASTT GSRLVSKNKW PLVFQALPPK FLEKNLAKAL DKEYLLPDIN ID ELEGVDN GCLIDEALLP LIIDEGKRKG EGNYRPRHLR DERKEETVQA FLDKYGYCNI PVGYEVHHIV PLSQGGADSI KNM IMLSIE HHERVTEAHA SYFKWRNT |
-Macromolecule #7: type I-F CRISPR-associated endoribonuclease Cas6/Csy4
| Macromolecule | Name: type I-F CRISPR-associated endoribonuclease Cas6/Csy4 / type: protein_or_peptide / ID: 7 Details: Molecular name from NCBI link:https://www.ncbi.nlm.nih.gov/protein/MBO6292765.1 Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 20.735873 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFSQILIIKP GTGISPNIII SEDIFPVLHS LFVEHDKKFG ITFPAYSFDK KGHLGNIIEV LSEDKEALAS LCLEEHLAEV TDYVKVKKE ITFTDDYVLF KRIREENQYE TTARRMRKRG HTELGRPLEM HIKKKNQQIF CHAYIKVKSA STGQSYNIFL A PTDIKHGS FSAYGLLRGD THA |
-Macromolecule #4: DNA (37-MER)
| Macromolecule | Name: DNA (37-MER) / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 11.27125 KDa |
| Sequence | String: (DT)(DG)(DC)(DT)(DA)(DA)(DG)(DC)(DG)(DC) (DA)(DC)(DC)(DT)(DA)(DA)(DT)(DT)(DT)(DC) (DC)(DT)(DG)(DA)(DC)(DG)(DG)(DC)(DA) (DA)(DT)(DC)(DC)(DG)(DC)(DA)(DC) |
-Macromolecule #5: DNA (5'-D(P*GP*TP*GP*CP*GP*GP*A)-3')
| Macromolecule | Name: DNA (5'-D(P*GP*TP*GP*CP*GP*GP*A)-3') / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 2.178447 KDa |
| Sequence | String: (DG)(DT)(DG)(DC)(DG)(DG)(DA) |
-Macromolecule #6: RNA (69-MER)
| Macromolecule | Name: RNA (69-MER) / type: rna / ID: 6 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Selenomonas sp. (bacteria) Selenomonas sp. (bacteria) |
| Molecular weight | Theoretical: 22.398293 KDa |
| Sequence | String: GUUUAGAAGG AUUGCCGUCA GGAAAUUAGG UGCGCUUAGC AGUGUACCGC CGGAUAGGCG GUUUAGAAG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 1.2 µm |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)