+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
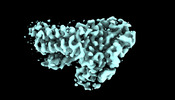
| Title | the structure of PsaQ | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | photosynthesis / chlorophyll / alloxanthin / growth phase | |||||||||
| Biological species |  Rhodomonas salina (eukaryote) Rhodomonas salina (eukaryote) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Zhang SM / Si L / Li M | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Growth phase-dependent reorganization of cryptophyte photosystem I antennae. Authors: Shumeng Zhang / Long Si / Xiaodong Su / Xuelin Zhao / Xiaomin An / Mei Li /  Abstract: Photosynthetic cryptophytes are eukaryotic algae that utilize membrane-embedded chlorophyll a/c binding proteins (CACs) and lumen-localized phycobiliproteins (PBPs) as their light-harvesting antennae. ...Photosynthetic cryptophytes are eukaryotic algae that utilize membrane-embedded chlorophyll a/c binding proteins (CACs) and lumen-localized phycobiliproteins (PBPs) as their light-harvesting antennae. Cryptophytes go through logarithmic and stationary growth phases, and may adjust their light-harvesting capability according to their particular growth state. How cryptophytes change the type/arrangement of the photosynthetic antenna proteins to regulate their light-harvesting remains unknown. Here we solve four structures of cryptophyte photosystem I (PSI) bound with CACs that show the rearrangement of CACs at different growth phases. We identify a cryptophyte-unique protein, PsaQ, which harbors two chlorophyll molecules. PsaQ specifically binds to the lumenal region of PSI during logarithmic growth phase and may assist the association of PBPs with photosystems and energy transfer from PBPs to photosystems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37674.map.gz emd_37674.map.gz | 5.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37674-v30.xml emd-37674-v30.xml emd-37674.xml emd-37674.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37674.png emd_37674.png | 57.2 KB | ||
| Filedesc metadata |  emd-37674.cif.gz emd-37674.cif.gz | 5.1 KB | ||
| Others |  emd_37674_half_map_1.map.gz emd_37674_half_map_1.map.gz emd_37674_half_map_2.map.gz emd_37674_half_map_2.map.gz | 140.7 MB 140 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37674 http://ftp.pdbj.org/pub/emdb/structures/EMD-37674 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37674 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37674 | HTTPS FTP |
-Related structure data
| Related structure data |  8wnwMC  8wm6C  8wmjC  8wmvC  8wmwC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_37674.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37674.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04004 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37674_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
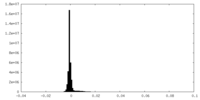
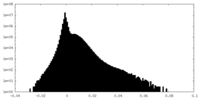
| Density Histograms |
-Half map: #1
| File | emd_37674_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
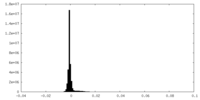
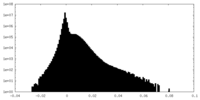
| Density Histograms |
- Sample components
Sample components
-Entire : pigment binding protein
| Entire | Name: pigment binding protein |
|---|---|
| Components |
|
-Supramolecule #1: pigment binding protein
| Supramolecule | Name: pigment binding protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Rhodomonas salina (eukaryote) Rhodomonas salina (eukaryote) |
-Macromolecule #1: PsaQ
| Macromolecule | Name: PsaQ / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhodomonas salina (eukaryote) Rhodomonas salina (eukaryote) |
| Molecular weight | Theoretical: 23.834393 KDa |
| Sequence | String: RTAAYVALAA ASAEAFSTPA LSGLKMAEPA QISRKDVLTT AAGAAIIAMP TLAGAASLDP KTGFPIQGGS REKLCGGSAS AGCQPMTQA ASILDKQRTV LAGKITVAAN KVPVLTAAVD KMKTAKKPKL DRDYVLRYSA LYLTTLVDAM EQYCLRDANG A KAAGGAGL ...String: RTAAYVALAA ASAEAFSTPA LSGLKMAEPA QISRKDVLTT AAGAAIIAMP TLAGAASLDP KTGFPIQGGS REKLCGGSAS AGCQPMTQA ASILDKQRTV LAGKITVAAN KVPVLTAAVD KMKTAKKPKL DRDYVLRYSA LYLTTLVDAM EQYCLRDANG A KAAGGAGL PKFKETLKPA SSSSLYSYVD TVKSGIAAVS AAAKAGDFDG VNKAAGDIKT AADSFLSTAN PPIIFN |
-Macromolecule #2: 1,2-DISTEAROYL-MONOGALACTOSYL-DIGLYCERIDE
| Macromolecule | Name: 1,2-DISTEAROYL-MONOGALACTOSYL-DIGLYCERIDE / type: ligand / ID: 2 / Number of copies: 1 / Formula: LMG |
|---|---|
| Molecular weight | Theoretical: 787.158 Da |
| Chemical component information |  ChemComp-LMG: |
-Macromolecule #3: CHLOROPHYLL A
| Macromolecule | Name: CHLOROPHYLL A / type: ligand / ID: 3 / Number of copies: 2 / Formula: CLA |
|---|---|
| Molecular weight | Theoretical: 893.489 Da |
| Chemical component information |  ChemComp-CLA: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 38675 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)