+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human Alpha-fetoprotein | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationprogesterone metabolic process / ovulation from ovarian follicle / small molecule binding / Post-translational protein phosphorylation / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / blood microparticle / endoplasmic reticulum lumen / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Liu N / Liu K / Wu C / Liu Z / Li M / Wang J / Wang HW | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2023 Journal: Nat Methods / Year: 2023Title: Uniform thin ice on ultraflat graphene for high-resolution cryo-EM. Authors: Liming Zheng / Nan Liu / Xiaoyin Gao / Wenqing Zhu / Kun Liu / Cang Wu / Rui Yan / Jincan Zhang / Xin Gao / Yating Yao / Bing Deng / Jie Xu / Ye Lu / Zhongmin Liu / Mengsen Li / Xiaoding Wei ...Authors: Liming Zheng / Nan Liu / Xiaoyin Gao / Wenqing Zhu / Kun Liu / Cang Wu / Rui Yan / Jincan Zhang / Xin Gao / Yating Yao / Bing Deng / Jie Xu / Ye Lu / Zhongmin Liu / Mengsen Li / Xiaoding Wei / Hong-Wei Wang / Hailin Peng /  Abstract: Cryo-electron microscopy (cryo-EM) visualizes the atomic structure of macromolecules that are embedded in vitrified thin ice at their close-to-native state. However, the homogeneity of ice thickness, ...Cryo-electron microscopy (cryo-EM) visualizes the atomic structure of macromolecules that are embedded in vitrified thin ice at their close-to-native state. However, the homogeneity of ice thickness, a key factor to ensure high image quality, is poorly controlled during specimen preparation and has become one of the main challenges for high-resolution cryo-EM. Here we found that the uniformity of thin ice relies on the surface flatness of the supporting film, and developed a method to use ultraflat graphene (UFG) as the support for cryo-EM specimen preparation to achieve better control of vitreous ice thickness. We show that the uniform thin ice on UFG improves the image quality of vitrified specimens. Using such a method we successfully determined the three-dimensional structures of hemoglobin (64 kDa), α-fetoprotein (67 kDa) with no symmetry, and streptavidin (52 kDa) at a resolution of 3.5 Å, 2.6 Å and 2.2 Å, respectively. Furthermore, our results demonstrate the potential of UFG for the fields of cryo-electron tomography and structure-based drug discovery. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33861.map.gz emd_33861.map.gz | 12.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33861-v30.xml emd-33861-v30.xml emd-33861.xml emd-33861.xml | 14.7 KB 14.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33861.png emd_33861.png | 80.8 KB | ||
| Masks |  emd_33861_msk_1.map emd_33861_msk_1.map | 12.9 MB |  Mask map Mask map | |
| Others |  emd_33861_half_map_1.map.gz emd_33861_half_map_1.map.gz emd_33861_half_map_2.map.gz emd_33861_half_map_2.map.gz | 11.9 MB 11.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33861 http://ftp.pdbj.org/pub/emdb/structures/EMD-33861 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33861 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33861 | HTTPS FTP |
-Validation report
| Summary document |  emd_33861_validation.pdf.gz emd_33861_validation.pdf.gz | 648.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33861_full_validation.pdf.gz emd_33861_full_validation.pdf.gz | 647.9 KB | Display | |
| Data in XML |  emd_33861_validation.xml.gz emd_33861_validation.xml.gz | 9.2 KB | Display | |
| Data in CIF |  emd_33861_validation.cif.gz emd_33861_validation.cif.gz | 10.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33861 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33861 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33861 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33861 | HTTPS FTP |
-Related structure data
| Related structure data |  7yimMC  7xgyC  8gvkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33861.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33861.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.0382 Å | ||||||||||||||||||||
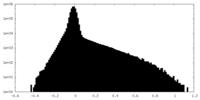
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_33861_msk_1.map emd_33861_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
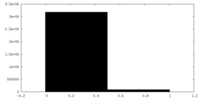
| Density Histograms |
-Half map: #1
| File | emd_33861_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
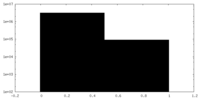
| Density Histograms |
-Half map: #2
| File | emd_33861_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
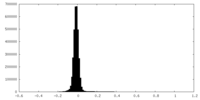
| Density Histograms |
- Sample components
Sample components
-Entire : Alpha-fetoprotein with no symmetry
| Entire | Name: Alpha-fetoprotein with no symmetry |
|---|---|
| Components |
|
-Supramolecule #1: Alpha-fetoprotein with no symmetry
| Supramolecule | Name: Alpha-fetoprotein with no symmetry / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 67 KDa |
-Macromolecule #1: Alpha-fetoprotein
| Macromolecule | Name: Alpha-fetoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.757406 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKWVESIFLI FLLNFTESRT LHRNEYGIAS ILDSYQCTAE ISLADLATIF FAQFVQEATY KEVSKMVKDA LTAIEKPTGD EQSSGCLEN QLPAFLEELC HEKEILEKYG HSDCCSQSEE GRHNCFLAHK KPTPASIPLF QVPEPVTSCE AYEEDRETFM N KFIYEIAR ...String: MKWVESIFLI FLLNFTESRT LHRNEYGIAS ILDSYQCTAE ISLADLATIF FAQFVQEATY KEVSKMVKDA LTAIEKPTGD EQSSGCLEN QLPAFLEELC HEKEILEKYG HSDCCSQSEE GRHNCFLAHK KPTPASIPLF QVPEPVTSCE AYEEDRETFM N KFIYEIAR RHPFLYAPTI LLWAARYDKI IPSCCKAENA VECFQTKAAT VTKELRESSL LNQHACAVMK NFGTRTFQAI TV TKLSQKF TKVNFTEIQK LVLDVAHVHE HCCRGDVLDC LQDGEKIMSY ICSQQDTLSN KITECCKLTT LERGQCIIHA END EKPEGL SPNLNRFLGD RDFNQFSSGE KNIFLASFVH EYSRRHPQLA VSVILRVAKG YQELLEKCFQ TENPLECQDK GEEE LQKYI QESQALAKRS CGLFQKLGEY YLQNAFLVAY TKKAPQLTSS ELMAITRKMA ATAATCCQLS EDKLLACGEG AADII IGHL CIRHEMTPVN PGVGQCCTSS YANRRPCFSS LVVDETYVPP AFSDDKFIFH KDLCQAQGVA LQTMKQEFLI NLVKQK PQI TEEQLEAVIA DFSGLLEKCC QGQEQEVCFA EEGQKLISKT RAALGV |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE / Support film - topology: HOLEY ARRAY |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 354264 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X